Efficacy and safety of APT036 versus simethicone in the treatment of functional bloating: a multicentre, randomised, double-blind, parallel group, clinical study
Introduction
Bloating is a common symptom reported by around 16% to 31% of the general population (1-4). In the absence of a consensus definition, bloating is generally considered to be the subjective sensation of increased abdominal pressure. The related term, abdominal distension, is the objective increase in abdominal girth which may accompany bloating (5-7).
Bloating is a heterogeneous condition and is a common complaint in individuals with functional gastrointestinal disorders (FGIDs) comprising irritable bowel syndrome (IBS), functional dyspepsia, and functional constipation (6). Under Rome III criteria, a diagnosis of functional bloating is made in patients with recurrent symptoms of bloating who do not meet the diagnostic criteria of IBS or other FGIDs (5,6).
Although the pathophysiology of bloating is not completely understood, multiple factors are known to be involved and their relative contribution varies between individuals. Potential mechanisms include increased luminal gas production, impaired gas handling and clearance, small intestinal distension due to excessive luminal fluid, small intestinal bacterial overgrowth (SIBO) or other changes in the gut microbiota, altered gut motility, visceral hypersensitivity, hard stool or constipation, food intolerance and carbohydrate malabsorption, abnormal abdominal-diaphragmatic muscle function, and altered pelvic floor function (5-7).
Functional bloating can arise without any predisposing factors and is unlikely to be completely resolved with medication and/or lifestyle modification such as a diet low in fermentable oligosaccharides, disaccharides, monosaccharides, and polyols (FODMAPs) in IBS. Medicinal treatment options include: stimulants of intestinal fluid secretion and motility—lubiprostone and linaclotide; antidepressants—citalopram and fluoxetine; the antibiotic rifaximin; probiotics such as Bifidobacterium bifidum MIMBb75, Lactobacillus plantarum 299v or combinations of multiple bacterial species, although their efficacy is inconclusive; prokinetics—prucalopride and formerly tegaserod which was withdrawn from the world market in 2007 due to the risk of serious cardiovascular adverse effects; antispasmodics—mebeverine and otilonium bromide; and gas-reducing agents e.g. simethicone (5-7).
Simethicone is an inert substance with antifoaming activity that reduces bloating, abdominal discomfort, and abdominal pain by dispersing and preventing the formation of mucus-surrounded gas pockets along the gastrointestinal tract (8). It was first approved for use by the US Food and Drug Administration in 1952 (9). Simethicone may act as a topical mucosal barrier providing protection against irritants such as gastric acid, biliary salts and acetylsalicylic acid (9,10). In the treatment of IBS, a meta-analysis showed that global symptoms and bloating were improved by the addition of simethicone to antispasmodic agents (11).
APT036 (Aprotecol®) contains xyloglucan which is extracted from the seeds of the tamarind tree Tamarindus indica. Xyloglucan has been approved in Europe (MED class III) for restoring the physiological functions of the intestinal walls. Available in capsule form for adults, and powder for paediatric use, xyloglucan has been specifically formulated to control and reduce gastrointestinal symptoms of varying aetiologies, such as abdominal tension and frequent faecal emissions. Xyloglucan’s ‘mucin-like’ molecular structure forms a bio-protective film on intestinal mucosa which thereby improves mucosal resistance to intestinal pathogens and helps to restore normal intestinal function (12). The Aprotecol formulation also includes the tyndallized lactic-bacterial strains Lactobacillus reuteri and Bifidobacterium brevis, which prevent and reduce symptoms in subjects with altered gut flora (13).
The current study aimed to evaluate the safety and efficacy of APT036 in adult patients with functional bloating, using simethicone as a comparator.
Methods
This multicentre, double-blind, randomised, parallel-group study was conducted at gastroenterology outpatient medical centres in Romania. Patients were enrolled by gastroenterology or internal medicine specialist physicians. The study was registered with EudraCT Number 2014-00556572.
Inclusion criteria were male or female patients between 18 and 65 years of age, of Caucasian race, with a diagnosis of functional bloating. Subjects were required to provide written informed consent to participate in the study prior to screening.
Subjects who met any of the following criteria were not eligible for study admission: pregnant or breastfeeding women; allergy to one of the product ingredients; impossibility to attend study visits; health status not allowing study participation; diabetic patients; patients treated with antibiotics or those using purgatives within two weeks prior to the hydrogen breath test.
Using a computer-generated randomisation scheme, subjects were assigned in a 1:1 ratio to receive APT036 or simethicone for 20 consecutive days. Treatments were administered orally each day according to the approved product label: 1 capsule 3 times/day. Patients and investigators were blinded to treatment. Treatment adherence was monitored by pill counts.
The evaluation period was 30 days and subjects attended five clinic visits: at baseline (Visit 1); after 2 days of treatment (Visit 2); after 10 days of treatment (Visit 3); after 20 days of treatment (Visit 4; end of treatment); at 10 days after the end of treatment (Visit 5; follow-up).
Patients’ demographic data and medical history were recorded at baseline. At baseline and end of treatment, subjects underwent a hydrogen breath test. At baseline and at each study visit including follow-up, the following assessments were performed: abdominal girth measurement; general medical investigation; clinical symptoms evaluation based on patient journals; concomitant medication evaluation; and safety assessment.
Patient data were collected using purpose-designed case report forms (CRF, see Supplementary Material).
Subjects were free to withdraw from the study at any time without providing a reason. Investigators could withdraw subjects if deemed appropriate for safety or ethical reasons or if the study was deemed detrimental to the well-being of the patient.
Study objectives
The primary objective of the study was to evaluate the safety of APT036 in adult patients with functional bloating.
The secondary objective of the study was to assess the clinical efficacy of APT036 versus simethicone in alleviating symptoms of functional bloating.
Study outcome measures
Safety was assessed by the occurrence of adverse events during the study (frequency, intensity, and relationship with study treatments) as reported by the patient or observed by the physician, and by the results of clinical parameters monitored and vital signs examined at each study visit.
Clinical efficacy was evaluated according to patient-reported symptom severity using the Likert scale and recorded in daily journals; by measuring patients’ abdominal girth at each study visit; and by the change between baseline and end of treatment in the hydrogen breath test.
The hydrogen breath test was performed before and after glucose administration. A total of five measurements were taken: one before ingesting glucose and four at 30-minute intervals for a 2-hour period after glucose ingestion. A positive hydrogen breath test was defined as a hydrogen gas elevation of 12 parts per million (ppm) at two time points (ideally for two consecutive measurements) within the 2 hours following the glucose-loading dose. Clinically, an increase of ≥12 ppm above basal on the glucose positive hydrogen breath test supports a diagnosis of SIBO.
Ethical considerations
The study was performed in accordance with Good Clinical Practice in conducting human clinical trials and the World Medical Association (WMA) Declaration of Helsinki regarding Ethical Principles for Medical Research Involving Human Subjects adopted during the 64th WMA General Assembly at Fortaleza in Brazil on October 2013. The study gained full approval from the România Academia de Stiinţe Medicale, Comisia Naţională de Bioetică a Medicamentului şi a Dispozitivelor Medicale on 3 February 2016 under approval number 7DM/03.02.2016.
The study was performed in compliance with the requirements of the National Agency of Medicine and Medical Devices of Romania and National Ethical Committee for Biomedical Research. The study gained full regulatory approval from the National Agency of Medicine and Medical Devices of Romania on 11 February 2016.
Statistical methods
Based on previous data showing an expected mean difference of at least 0.26 and a standard deviation of 0.41768 between any two groups for ‘duration of bloating and distension in adult patients’, it was calculated that at least 54 randomly selected subjects per group (108 subjects in total) were required to ensure a power of 0.90 at a 5% significance level for comparisons of safety and efficacy between APT036 and simethicone.
All subjects who received at least one dose of APT036 or simethicone were included in the safety analysis. All subjects who received at least one dose of APT036 or simethicone were included in the intention-to-treat efficacy analysis.
Results were summarized using descriptive statistics: mean ± standard deviation (SD) for continuous variables and absolute (n) and relative (%) frequency counts for categorical variables.
To determine whether APT036 and simethicone were effective in alleviating the symptoms of functional bloating, an initial paired t-test was conducted to measure whether a statistical difference existed between baseline and different time points (e.g., study visits) in the entire patient population, without differentiating between study arms. Exploratory statistical tests were then performed to test whether APT036 and simethicone differed in their ability to reduce the symptoms of functional bloating. Depending on the type of data, the analyses performed included t-test, Mann–Whitney U, χ2, and Wilcoxon signed-rank tests.
Differences in group scores between and among clinical variables were calculated using analysis of variance (ANOVA). Clinical symptoms and signs of functional abdominal bloating were associated with variables such as age, gender, treatment dose, or other recorded variables. Depending on the type of associated variables, correlation estimates were based on Pearson correlation coefficient, Spearman’s rank correlation coefficient, and/or Kendall tau rank correlation coefficient.
Clinical study protocol
The protocol summary is available as supplementary information (Tables S1,S2).

Full table

Full table
Results
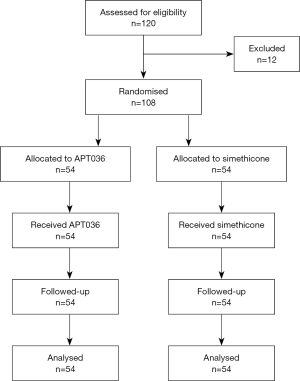
The study took place between 11 February and 10 September 2017. A total of 108 patients, enrolled at six gastroenterology outpatient medical centres in Romania (Bucharest: 4; Oradea: 1; Timisoara: 1), were randomised to receive APT036 (n=54) or simethicone (n=54). Patients’ demographic and baseline characteristics are summarised in Table 1. All patients completed the study (Figure 1) and their data were analysed for safety and efficacy.

Full table
No adverse events, serious adverse events, or serious unexpected adverse reactions were reported with APT036 or simethicone by patients or investigators during the study.
There were no significant differences between APT036 and simethicone in the evolution of clinical parameters and vital signs from baseline to Day 30 (Table 2).
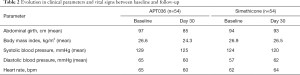
Full table
Combined assessment of the entire study population, without differentiating between APT036 and simethicone treatment groups, indicated statistically significant reductions between baseline and Day 2 in the clinical symptom of ‘distention’; statistically significant reductions between baseline and Days 10 and 20 in all clinical symptoms; and statistically significant reductions between Day 20 and Day 30 in the clinical symptoms of ‘distension’ and ‘flatulence’ (Table 3).
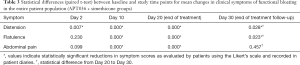
Full table
Comparing baseline with Day 30, there were statistically significance differences in favour of APT036 over simethicone in the number of subjects with symptoms of abdominal distension (P=0.008; Figure 2A) and flatulence (P=0.010; Figure 2B). Although the evolution from baseline to Day 30 in the number of subjects with abdominal pain also favoured APT036 (46 to 0 patients versus 35 to 5 patients with simethicone; Figure 2C), the difference between treatment groups did not reach statistical significance (P>0.05).
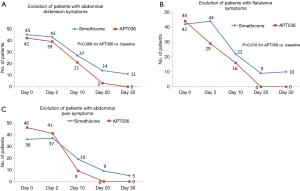
At baseline, the hydrogen breath test indicated the presence of SIBO in all subjects in both study arms (Table 4). Hydrogen gas elevation above basal levels after glucose administration was 14.3 ppm in both the APT036 and simethicone treatment groups, fulfilling the criteria for a diagnosis of SIBO. At the end of treatment (Day 20), all subjects in both treatment arms showed reduced hydrogen gas production. Mean peak hydrogen gas elevation was 8.8 ppm above basal with APT036 and simethicone (i.e., below the threshold for a diagnosis of SIBO), with no statistically significant difference between treatment groups.

Full table
Discussion
This multicentre, randomised, double-blind, parallel-group study was conducted to compare the safety and efficacy of APT036 and simethicone in patients with functional bloating. Both formulations were well tolerated by study subjects. With regard to the primary outcome measure, no adverse events, serious adverse events or serious unexpected severe adverse reactions were reported by patients or investigators throughout the study. Vital signs examined at baseline and follow-up were within normal ranges at each time point.
Efficacy analysis on clinical symptoms of functional bloating showed a better evolution with APT036 than simethicone for multiple symptoms. Compared with simethicone, APT036 significantly reduced abdominal distension (P=0.008) and flatulence (P=0.010) from baseline to the follow-up visit. APT036 also reduced symptoms of abdominal pain over the 30-day observation period compared with simethicone, although the difference was not statistically significant.
Hydrogen breath testing provides a safe, inexpensive, and non-invasive alternative to jejunal aspiration culture for the diagnosis of SIBO (14). Moreover, it may represent a more inclusive definition of SIBO because it is likely to include cases of distal small-bowel bacterial overgrowth and pathologic bacterial strains not identified by culturing techniques. The hydrogen breath test performed at baseline confirmed SIBO in all subjects (increase of >12 ppm above basal following glucose ingestion). Both APT036 and simethicone reduced patients’ production of hydrogen gas. At the end of treatment on Day 20, mean hydrogen breath test results in both study arms were below the threshold for a SIBO diagnosis (<12 ppm above basal after glucose ingestion), with no significant difference between APT036 and simethicone.
Disruption of intestinal epithelial barrier function is associated with several diseases including inflammatory bowel disease, IBS and celiac disease (15). In addition, the integrity of other mucosal barriers such as the respiratory epithelial barrier is an important defence against inflammatory and infectious diseases, e.g., chronic obstructive pulmonary disease, acute respiratory distress syndrome, and asthma (16,17). Non-pharmacological approaches such as xyloglucan, with demonstrated protective barrier properties, offer an alternative approach for managing a range of diseases characterised by mucosal disruption. In clinical trials, xyloglucan has been shown to reduce symptoms of gastroenteritis in children and adults (18,19), symptoms of rhinosinusitis (20), and dry eye syndrome (21). Treatment of IBS patients with a similar mucosal protector containing film-forming reticulated proteins plus oligo- and polysaccharides relieved abdominal pain and flatulence (22). In placebo-controlled trials, reticulated proteins were also effective in treating urinary tract infections (23,24). In a pilot study, APT198K (xyloglucan plus heat-killed Lactobacillus reuteri SGL01 and Bifidobacterium brevis SGB01) was superior to a lactase dietary supplement in reducing the mean duration of crying per episode in 46 children with infantile colic (25). The current study of APT036 extends evidence for the safety and efficacy of xyloglucan-containing medical devices, with demonstrated protective barrier properties, to the treatment of functional bloating.
A major limitation of randomized clinical trials is their restriction to interventions that are meant to have a positive treatment effect. Another limitation relates to the difficulty in interpreting or generalizing the results because the studied population is not wholly representative of the population treated in usual practice. Further studies of APT036 in patients with various comorbidities are required. The limitations of clinical trials also include the specificity of the question to be answered. Indeed, the narrow perspective of many trials excludes important information related to the consequences of the intervention on quality of life, treatment satisfaction or costs. A solution consists of developing a disease management approach that involves implementation of real-life studies performed in thousands of patients and with a long duration of follow-up.
In conclusion, APT036 had a good safety profile and was significantly superior to simethicone in relieving symptoms of functional bloating, namely abdominal distension and flatulence.
Acknowledgements
Editorial assistance in the preparation of this article was provided by Robert Furlong and Kerry Dechant of Content Ed Net, with funding from Noventure SL, Barcelona, Spain.
Funding: The study was sponsored by Novintethical Pharma SA (Pambio-Noranco, Switzerland).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study gained full approval from the România Academia de Stiinţe Medicale, Comisia Naţională de Bioetică a Medicamentului şi a Dispozitivelor Medicale on 3 February 2016 under approval number 7DM/03.02.2016, and full regulatory approval from the National Agency of Medicine and Medical Devices of Romania on 11 February 2016.
References
- Talley NJ, Boyce P, Jones M. Identification of distinct upper and lower gastrointestinal symptom groupings in an urban population. Gut 1998;42:690-5. [Crossref] [PubMed]
- Sandler RS, Stewart WF, Liberman JN, et al. Abdominal pain, bloating, and diarrhea in the United States: prevalence and impact. Dig Dis Sci 2000;45:1166-71. [Crossref] [PubMed]
- Tuteja AK, Talley NJ, Joos SK, et al. Abdominal bloating in employed adults: prevalence, risk factors, and association with other bowel disorders. Am J Gastroenterol 2008;103:1241-8. [Crossref] [PubMed]
- Jiang X, Locke GR 3rd, Choung RS, et al. Prevalence and risk factors for abdominal bloating and visible distention: a population-based study. Gut 2008;57:756-63. [Crossref] [PubMed]
- Seo AY, Kim N, Oh DH. Abdominal bloating: pathophysiology and treatment. J Neurogastroenterol Motil 2013;19:433-53. [Crossref] [PubMed]
- Iovino P, Bucci C, Tremolaterra F, et al. Bloating and functional gastro-intestinal disorders: where are we and where are we going? World J Gastroenterol 2014;20:14407-19. [Crossref] [PubMed]
- Foley A, Burgell R, Barrett JS, et al. Management strategies for abdominal bloating and distension. Gastroenterol Hepatol (N Y) 2014;10:561-71. [PubMed]
- Ebadi M. Desk Reference of Clinical Pharmacology, Second Edition. Boca Raton, FL, USA: CRC Press; 2011.
- MedicineNet.com. Simethicone. Available online: [Accessed 16 March 2018].https://www.medicinenet.com/simethicone/article.htm
- Meier R, Steuerwald M. Review of the therapeutic use of simethicone in gastroenterology. Schweizerische Zeitschrift fur GanzheitsMedizin 2007;19:380-7. [Crossref]
- Martínez-Vázquez MA, Vázquez-Elizondo G, González-González JA, et al. Effect of antispasmodic agents, alone or in combination, in the treatment of irritable bowel syndrome: systematic review and meta-analysis. Rev Gastroenterol Mex 2012;77:82-90. [Crossref] [PubMed]
- Piqué N, Gómez-Guillén MDC, Montero MP. Xyloglucan, a plant polymer with barrier protective properties over the mucous membranes: an overview. Int J Mol Sci 2018;19. [Crossref] [PubMed]
- Noventure. Aprotecol® package insert. Available online: (2017 accessed 13 March 2018).http://noventure.com/aprotecolr-drops
- Erdogan A, Rao SS, Gulley D, et al. Small intestinal bacterial overgrowth: duodenal aspiration vs glucose breath test. Neurogastroenterol Motil 2015;27:481-9. [Crossref] [PubMed]
- König J, Wells J, Cani PD, et al. Human intestinal barrier function in health and disease. Clin Transl Gastroenterol 2016;7:e196. [Crossref] [PubMed]
- Brune K, Frank J, Schwingshackl A, et al. Pulmonary epithelial barrier function: some new players and mechanisms. Am J Physiol Lung Cell Mol Physiol 2015;308:L731-45. [Crossref] [PubMed]
- Gon Y, Hashimoto S. Role of airway epithelial barrier dysfunction in pathogenesis of asthma. Allergol Int 2018;67:12-7. [Crossref] [PubMed]
- Pleșea Condratovici C, Bacarea V, Piqué N. Xyloglucan for the treatment of acute gastroenteritis in children: results of a randomized, controlled, clinical trial. Gastroenterol Res Pract 2016;2016. [Crossref] [PubMed]
- Gnessi L, Bacarea V, Marusteri M, et al. Xyloglucan for the treatment of acute diarrhea: results of a randomized, controlled, open-label, parallel group, multicentre, national clinical trial. BMC Gastroenterol 2015;15:153. [Crossref] [PubMed]
- Allegrini A, Pavone D, Carluccio F. A randomized controlled trial comparing a xyloglucan-based nasal spray with saline in adults with symptoms of rhinosinusitis. Curr Med Res Opin 2018;34:377-85. [Crossref] [PubMed]
- Rolando M, Valente C. Establishing the tolerability and performance of tamarind seed polysaccharide (TSP) in treating dry eye syndrome: results of a clinical study. BMC Ophthalmol 2007;7:5. [Crossref] [PubMed]
- Alexea O, Bacarea V, Piqué N. The combination of oligo- and polysaccharides and reticulated protein for the control of symptoms in patients with irritable bowel syndrome: Results of a randomised, placebo-controlled, double-blind, parallel group, multicentre clinical trial. United European Gastroenterol J 2016;4:455-65. [Crossref] [PubMed]
- García-Larrosa A, Alexe O. Efficacy and safety of a medical device versus placebo in the early treatment of patients with symptoms of urinary tract infection: a randomized controlled trial. Clin Microbiol 2016;5:233. [Crossref]
- Salvatorelli N, García-Larrosa A, Allegrini A, et al. A new approach to the treatment of uncomplicated cystitis: results of a randomized placebo-controlled clinical trial. Urol Int 2016;97:347-51. [Crossref] [PubMed]
- Vandenplas Y, Bacarea A, Marusteri M, et al. Efficacy and safety of APT198K for the treatment of infantile colic: a pilot study. J Comp Eff Res 2017;6:137-44. [Crossref] [PubMed]
Cite this article as: Burta O, Iacobescu C, Mateescu RB, Nicolaie T, Tiuca N, Pop CS. Efficacy and safety of APT036 versus simethicone in the treatment of functional bloating: a multicentre, randomised, double-blind, parallel group, clinical study. Transl Gastroenterol Hepatol 2018;3:72.