Surgical treatment of gastrointestinal stromal tumors of the duodenum: a literature review
Introduction
Gastrointestinal stromal tumours (GIST) are the most common type of mesenchymal tumours found in the gastrointestinal tract (1). For years, GISTs have been defined as smooth gastrointestinal muscle tumors and have been called under various names and often misdiagnosed as leiomyomas, schwannomas and sarcomas (2). Currently, the distinctive feature of GIST is the mutation in the c-kit protooncogene leading to gain-of-function and subsequent cell proliferation (3-5). GISTs are rare, with relative annual incidence of 14.5 per million and prevalence of 129 per million (6). GIST may occur anywhere in the digestive tract, but are more frequently located in the stomach (60–70%) and midgut (25%) and less often in colon and rectum (5–10%) (7). Extravisceral GIST occurs in less than 10% of patients, most frequently in mesentery, intrabdominal, pelvis and retroperitoneal space (7). Duodenal GISTs (dGIST) represent only 4–5% of all GISTs, but accounted for 6–21% of surgical resected ones (8-10). The complex anatomy of the duodeno-pancreatic region can make their diagnosis and treatment extremely challenging. Anatomical closeness to noble structures (i.e., to the head of the pancreas, kidney and biliary structures) can lead to misdiagnosis and inappropriate management (11). Additionally, several factors complicate the management of dGISTs such as the relative lack of experience, the ambiguous clinical manifestations that often mimic a wide range of clinical conditions, the anatomical complexity and the lack of consensus on treatment.
The publication of solid research findings (3,12) that characterize the pathogenesis and histology of GIST have created a discontinuity in the terminology used to describe this entity. Due to the unclear terminology of scientific literature published before 2000, it is very difficult to analyse and interpret the previous reports, but the researches published since then has significantly increased (4).
Herein, we aimed to perform a state-of-the-art review of available English literature to improve the understanding of dGIST and to outline the best options for surgical treatment.
Methods
Search strategy
Comprehensive research has been conducted to identify all clinical studies that report the occurrence of dGIST. The search was carried out on the electronic databases MEDLINE, Scopus, EMBASE and Cochrane Libraries. The research strategy included all studies published after 2000 and focused on relevant articles using the following keywords: “gist”, “duodenal”, “stromal gastrointestinal cancer”, “surgery” and associated free terms. No language restrictions have been imposed to limit results.
Inclusion criteria
Studies were selected for consideration in this analysis provided they reported cases with dGIST. In order to select only those studies that certainly dealt with dGIST, only those studies in which the immunohistochemical analysis of the cKIT was reported and were positive were included.
Study selection
The inclusion criteria were used to examine the title, abstracts and full texts of the studies obtained from the research results. Two reviewers independently examined all citations on titles and abstracts produced by the search strategy and retrieved all potentially relevant reports. Two other independent readers then screened the full text of the studies to identify the entries that met the inclusion criteria. Any disagreements have been resolved by consensus. Differences in data extraction have been resolved by consensus, with references to the original article.
Results
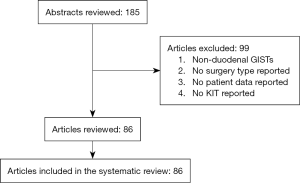
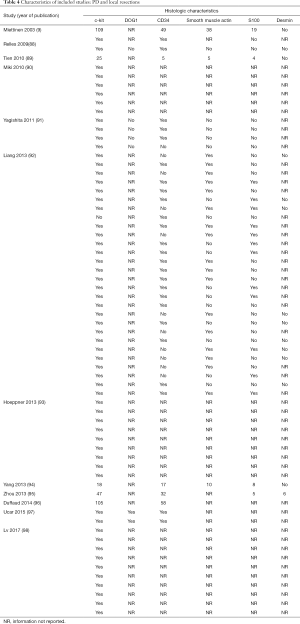
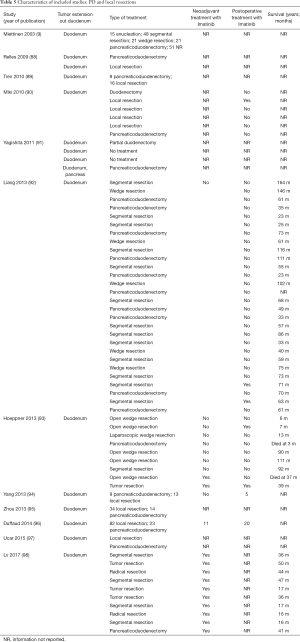
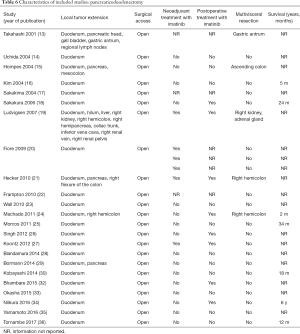
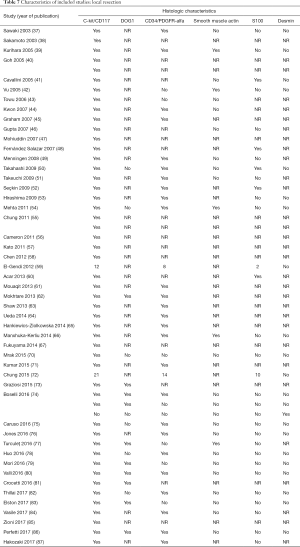
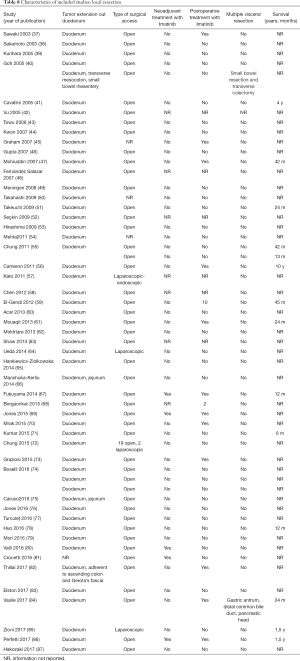
The initial search produced 185 potentially relevant articles (Figure 1) (Tables 1,2,3,4,5) that were suitable for subsequent screening. The titles and abstracts of these articles were screened to determine the relevance and eligibility for inclusion—53 papers were excluded for different reasons: non-duodenal GISTs, no surgery type reported, and no patient data reported leaving 132 studies for inclusion in the present review. Of them, 46 studies did not report the data about cKIT and were excluded thus leaving 86 studies included in the analysis. The patients excluded for non-duodenal GIST were 7,142, those excluded for no surgery type reported were 1,039 and 1,002 patients were excluded because of no cKIT reported. The characteristics of the included studies have been summarized in Tables 1,2,3,4,5. All studies were published between 2001 and 2017. Eighty-two papers with 539 cases reported data on the signs and symptoms at the time of hospital admission, whereas in four studies there were no data. Upon admission, the most common symptoms were GI bleeding and melena, followed by abdominal pain and discomfort. Symptoms of fatigue, fainting, vomiting, and ankle edema were also reported but occurred far less frequently. In the majority of cases dGISTs were located in the second portion of the duodenum. Several cases reported dGIST localization in both the second and third portions of the duodenum.
Full table
Full table
Full table
Full table
Full table
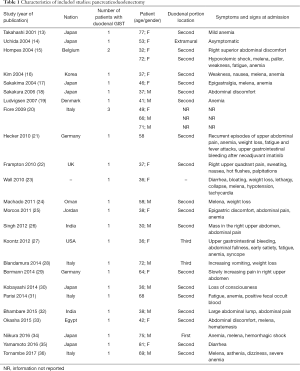
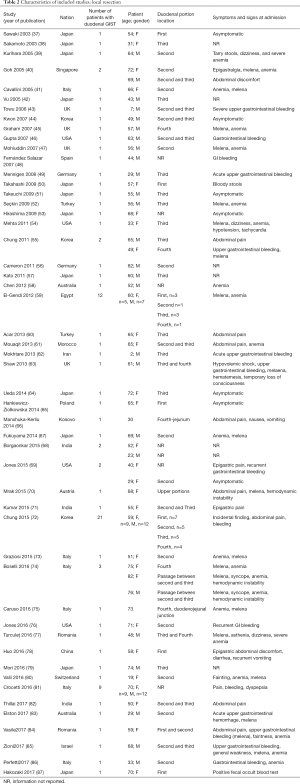
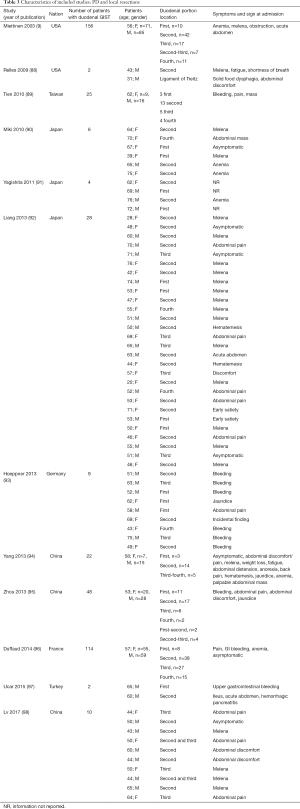
The included studies summoned a total of 549 patients with dGIST—27 patients treated with pancreatoduodenectomy (PD) (Table 1), 96 patients treated with local resection (Table 2), and 426 patients treated with either PD or segmental/wedge resections (Tables 3,4,5).
Among patients treated with PD (Tables 1,6), the majority were females (n=14, 52%; males: n=11, 41%; not reported: n=2, 7%), with a median age of 50 years (Table 1). Most patients presented with symptoms of anemia (n=11, 40%), melena (n=8, 27%), fatigue (n=4, 15%), and weight loss (n=4, 15%). However, in 3 patients the information was not reported. For most patients, the dGIST were located in the second portion of the duodenum (first: n=1, 4%; second: n=19, 70%; third: n=1, 4%; not reported: n=5, 18%) (Table 1). Four studies (13,19,21,25) reported dGIST tumors that extended beyond the duodenum. All PDs were performed by open procedure. While some patients received imatinib as a neoadjuvant treatment (n=7, 26%), a greater proportion received the treatment post-operatively (n=9, 33%) (Table 6). For most patients, no long term survival data were reported (n=21, 75%). Notably, the PD studies reported histological information on KIT, DOG1, or CD34.
Full table
Among patients treated with local resection (Tables 2,7) there was a slight preponderance of males (n=51, 53%; females: n=45, 47%) with a median age of 57.3 years (Table 2). Upon admission, patients most commonly presented with nausea (n=1, 1%), abdominal pain (n=8, 8%), undetermined pain (n=18, 19%), melena (n=20, 21%), dizziness (n=3, 3%), and/or GI bleeding (n=14, 16%). No data on presenting signs and symptoms was available for 7 patients (Table 2).
Full table
Within this group, most dGIST were located in the second and third portions of the duodenum (first: n=16, 17%; second: n=29, 30%, third: n=32, 33%; fourth: n=12, 12%; not reported: n=7, 7%), with tumors extending beyond the duodenum in four cases. For all local resections reported, surgical access was predominantly achieved by either open (n=89, 92%) or laparoscopic procedure (n=5, 5%). Patients treated with a local resection received less neoadjuvant (n=13, 13%) and post-operative (n=22, 23%) treatment with imatinib (Table 8), as compared to patients treated with a pancreaticoduodenectomy (Table 6), and four patients underwent multiple visceral resections.
Full table
Of the patients whose histological or molecular data were provided (Table 7), most tested positive for cKIT (n=85, 46%), CD34 (n=48, 26%) and DOG1 (n=20, 11%). A minority of patients tested positive for either smooth muscle actin (n=5, 3%), S100 (n=7, 4%) or desmin (n=1, 1%).
Four hundred twenty-six patients were treated with either PD or segmental/wedge resections. Within this group, there was preponderance of males (n=218, 51%) vs. females (n=183, 43%), not reported (n=25, 6%). At the time of admission, patients frequently presented with symptoms of bleeding (n=137, 32%), abdominal pain or discomfort (n=258, 60%) and melena (n=235, 55%). Five patients were asymptomatic (Tables 3,4,5). dGISTs were predominantly located in the second and third portions of the duodenum in this group (first: n=47, 11%; second: n=164, 38%; third: n=83, 19%; fourth: n=41, 9%). In many cases the tumors extended into multiple portions of the duodenum simultaneously. All of these studies reported data on cKIT and several studies reported data on CD34 and DOG1.
Within this cohort the most common type of surgical treatment was local resection (n=151, 35%), followed by PD (n=92, 21%) and segmental (n=97, 15%) or wedge resections (n=32, 7%). No data on the type of surgical treatment were available for 51 patients. Within this group of patients, both neoadjuvant and post-operative treatment with imatinib were rarely applied (neoadjuvant treatment: n=23, 5%; adjuvant treatment: n=30, 7%). Most patients received no imatinib treatment prior to surgery (n=160, 38%) or post-operatively (n=149, 35%).
Surgical treatment
There are three main surgical options: pancreatoduodenectomy (PD) (10,24), wedge resection (40,60,63,97) and segmental resection. PD is indicated in the cases with involvement of major duodenal papilla, pancreas or pancreatic duodenal wall and is required in 20–40%. Based on the literature, the following options for surgical reconstruction according to the localization and extent of duodenal resection are available:
First and second duodenal portions:
- wedge resection with primary transverse closure of duodenum (39) or retrocolic Roux-en-Y loop to cover a large defect (97);
- segmental resection, closure of distal stump and duodenojejunostomy by retrocolic Roux-en-Y loop (99);
- segmental resection with antrectomy with side-to-side posterior or Roux-en-Y gastrojejunostomy (55,59);
- subtotal resection with side-to-side duodenojejunostomy (100);
- sleeve resection with gastroduodenostomy (the case with situs inversus totalis) (82);
- ampullectomy with sphincteroplasty (55).
Third and fourth duodenal portions:
- segmental resection with closure of distal stump and side-to-side (93) or end-to-side/end-to-end duodenojejunostomy (62,92);
- segmental resection, end-to-end anastomosis, pylorus closure and gastroenterostomy with/without feeding jejunostomy (77);
- segmental resection, closure of distal stump near leg. Treitz and end-to-side duodenojejunostomy with first jejunal loop (99);
- segmental resection with end-to-side or end-to-end duodenojejunostomy to the left of the superior mesenteric vessels (66,72,75) segmental resection, closure of distal stump on the right of superior mesenteric vessels, papilloplasty, end-to-side duodenojejunostomy, transcystic tube and transjejunal tubes draining the main pancreatic duct and decompressing duodenum (38);
- segmental resection of third and fourth part of duodenum, closure of second portion stump and side-to-end duodenojejunostomy (49);
Discussion
Duodenal GIST is typically seen in individuals between the ages of 60–70 years old. There were few papers reporting an occurrence of dGIST in children and teenagers, all of which manifested with severe bleeding (80,97,101). The median age of the patients with dGIST is 56 years with a slight preponderance of males (54% vs. 46%) (1,5).
Risk factors
Most cases are sporadic, whereas familial clustering is reported in only 1.5–4% of the cases (69,102). Familial GIST is an autosomal dominant condition caused by germline mutations of cKIT or PDGFRA and manifests at earlier age. GIST may be part of Carney’s triad (gastric GIST, paraganglioma, and pulmonary chondroma) or Carney-Stathakis syndrome, which are caused by germline mutations in SDH (89). It is a well-known fact that von Recklinghausen neurofibromatosis type 1 (NF 1) is associated with an increased risk for GIST. GISTs in NF 1 occur in 6–7%, affect duodenum in 22–31%, and tend to be smaller, multiple and with lower mitotic count and occur at younger age (89,103,104). Hakozaki et al. described a case of dGIST and rectal cancer in a patient with NF 1 (87). Malignant carcinoid of ampulla Vateri and bilateral feochromocitoma have been also described with low responses to imatinib (105). Few papers reported extremely rare combination with pancreatic neuroendocrine neoplasia (64) and somatostatinoma in NF 1 (35) and Brunner’s gland cysts (78).
Localization
Most dGISTs develop in the second (59–63%) and third portion (22%) of duodenum (55,106), whereas the first and forth are less frequently affected. GIST of ampulla Vateri is extremely rare and according to the review of Kobayashi et al. only 12 cases have been described till 2014 (30). In all cases pain, jaundice and melena were the most frequent symptoms. Despite its rarity, this localization requires meticulous differential diagnosis (neuroendocrine tumors, carcinoma, paraganglioma) because usually the radical treatment requires PD (107).
Histopathology and molecular characteristics
Histologically, dGISTs do not differ from other GIST localizations. The most frequently reported pattern was spindle cell (67%), but epithelioid (11%), pleomorphic, mixed (22%), hemangioma-like or hemangiopericytoma-like patterns can also be seen (9,13,55,93,99,101,108-114). Immunohistochemistry staining of the specimens revealed the following distribution of the markers: CD117 (c-kit) (92–100%), and less frequently CD34 (54–70%), smooth muscle actin (20–30%), S-100 protein (10–20%), and neurofilament 68 (14%), DOG1 protein (6%) (9,12,65,94,115). Lack of DOG-1 expression was associated with poor prognosis in a recent study with 332 patients (93). The differential diagnosis includes fibromatosis (110), schwannomas, leiomyomas, inflammatory fibroid tumors, solitary fibrous tumor, mesenteric sclerosing fibrotic lesions, sarcomas, metastasis from malignant melanoma, glomus tumors, paragangliomas, ectopic pancreas (65,92,98).
At the molecular level, approximately 96% of GISTs have cKIT (CD117) mutation, typically in exon 11, but mutations in exons 9, 13, and 17 may also occur (101). Exon 11 mutations carry a better prognosis and respond well to standard dose (400 mg/dayly) of imatinib (116). Some authors reported worse outcomes in exon 9 mutation (102). Approximately 8% of the cases have PDGRFA mutation, which is mutually exclusive with cKIT but is associated with longer relapse-free interval (43,59,116). Some PDGFRA are resistant to imatinib (65). The so-called wild type is observed in 10–15% of the cases and in 90% of children. It is caused by germline mutations in BRAF V600E, RAS family or succinate dehydrogenase subunits (SDH A-D). GISTs in NF1 are usually wild type with possible implication of RAS-MAPK pathway (104,117-119). This subset of GISTs is relatively resistant to the treatment with imatinib. Further large studies are needed to elucidate the individual prognosis according to the mutational status.
Clinical manifestation
Approximately 70% of the cases manifest with symptoms, while 21% are found incidentally and 10% on autopsy (120). Our analysis revealed that in contrast to the other localizations the most frequent manifestation of dGIST is the upper gastrointestinal bleeding, which is in accordance with the literature (21,74,121). Constant/intermittent dull pain/discomfort or abdominal palpable mass are less frequent initial symptoms (10,32,71). Rarely, however, dGISTs may have various initial presentations leading to misdiagnosis. Millonig et al. reported extrahepatic cholestasis and Takotsudo cardiomyopathy in a patient with undiagnosed NF1 (122). The extremely rare dGIST of ampulla Vateri can also manifest with jaundice (30). In certain cases, extramural growth may mimic pancreatic head tumor (26,44,84,97), large duodenal cyst (61), bleeding or uncomplicated duodenal diverticula (33, 46). Wall et al. reported a rare case with duodenal-jejunal intussusception (23). Lin et al. described two cases with bleeding small dGISTs (<2 cm) initially misdiagnosed as hemobilia (123). A case mimicking refractory peptic ulcer treated for 8 years was also described (76), even hypercalcemia due to elevated serum calcitriol in metastatic disease as well (124).
Risk stratification
The recurrence risk and survival outcomes for dGIST are difficult to be determined based solely on the histopathological characteristics. Multiple factors have been proposed as predictive of survival outcomes, including tumor location and size, mitotic rate, kinase mutational status, and incidence of tumor rupture. However, tumor size and mitotic rate are the two most widely accepted risk indicators (125,126).
The Fletcher’s and subsequently the modified National Institute of Health classification are the most popular risk classifications (95). Miettinen et al., based on the follow-up of 140 cases with dGIST, proposed a distinct risk stratification of dGIST in which group 1 (<2 cm and <5 mitoses) is considered benign, whereas group 6 (>5 cm and >5 mitoses) carries an extremely poor prognosis with 86% mortality (18/21) within 21 months (9). The overall mortality in their series was 34%. This classification was externally validated by the French Sarcoma group in a series with 114 patients (119). The overall doubling time for dGIST is 17 months in comparison to leiomyoma (231 months). According to the risk group the doubling time is 24, 17 and 4 months in low, intermediate and high risk dGISTs (115).
Generally, there are contradictory results in the literature regarding the prognosis of dGIST in comparison to the other localizations. Certain authors reported worse outcome in comparison to small bowel and gastric GISTs (43), whereas others reported similar prognosis with small bowel localization. The more recent, population-based study of Guller et al. reported similar survival in gastric, duodenal and small bowel GISTs in contrast to colonic and extravisceral localizations (127). This finding was corroborated after subgroup analysis of two periods (1998–2004 vs. 2005–2011) thus eliminating a possible cofounding factor associated with the implementation of imatinib therapy.
Diagnosis
Although it may be straightforward in most cases, certain considerations should be kept in mind due to their occasionally misleading manifestation (128). Differential diagnosis includes adenocarcinoma, endocrine tumors, benign tumors and rare entities such as intrabdominal fibromatosis (110), ectopic pancreas (129), and Brunner’s gland cysts (78).
Because most of dGISTs present with acute bleeding or chronic anemia endoscopic evaluation of upper gastrointestinal tract should be the first step (101). It allows for biopsy and can be also therapeutic. In case of failure, transarterial embolization is a method of choice, either as definitive hemostasis or as a bridging procedure before surgical intervention (39). Occasionally, endoscopy may be misleading, especially in small intramural lesions without mucosal involvement (ulceration or central depression) or located near the papilla Vateri. In such cases of diagnostic uncertainty, endoscopic ultrasound (EUS) is an invaluable modality. Usually dGISTs appears as hypoechoic and well-vascularized lesion. Two small series reported a significant correlation between the presence of intratumoral vessels on contrast-enhanced or color Doppler EUS and the malignant potential of dGISTs (130,131). EUS provides a precise evaluation of the size, border, layer of origin, echogenicity and heterogeneity of the lesions thus facilitating the differential diagnosis (lipomas, hemangiomas, ectopic pancreas, and cysts) and decision-making process (112). Additionally, EUS allows also fine needle or trucut biopsy with 100% specificity and 84% sensitivity (92,112,131,132). Percutaneous biopsy should be avoided when possible because of the risk for tumor spillage and dissemination.
Abdominal US is useful screening tool in the cases with dull pain in upper abdomen, but computed tomography (CT) and magnetic resonance imaging (MRI) are mandatory to make an exact staging and preoperative planning of surgery (107,113). On CT dGISTs appear as well-defined, heterogeneously enhanced, hypervascular mass with prominent feeding arteries and intra- or extramural growth (107,114). In contrast, the ectopic pancreas has intraluminal growth and ill-defined border with enhancement of the overlying mucosa (129). The periampullary pancreatic cancer is usually hypodense on arterial phase with concomitant pancreatic duct dilatation, periampullary neuroendocrine tumors reveal hypervascular enhancement, calcifications, lack of ductal obstruction, central necrosis and cystic degeneration, whereas solid periampullary tumors demonstrate heterogeneous hypoenhancement in both phases (114). Fluorodeoxyglucose positron emission tomography (FDG-PET) is not routine tool but can be useful to monitor the effect from imatinib treatment and follow-up (133,134).
Surgical treatment
The ESMO guideline suggests that tumors under 2 cm has low aggressive behaviour and therefore could be followed annually by endoscopic ultrasound, “although an evidence-based optimal surveillance policy is lacking” (109). In contrast to the other localizations, in dGIST there is no uniformly adopted surgical strategy because of the low incidence, lack of enough experience, and the complex anatomy of the duodenum. Therefore, individually tailored surgical approach is recommended. In fact, there are three main surgical options: pancreatoduodenectomy (PD), wedge resection and segmental resection.
PD is indicated in the cases with involvement of major duodenal papilla, pancreas or pancreatic duodenal wall and is required in 20–40% (101,135). In some cases, it can be performed successfully in emergency setting due to life-threatening bleeding (10,24,111) even with second-stage pancreatojejunostomy (17). Tien et al. found that size >5 cm and preoperative diagnosis of dGIST were more frequently treated by PD (89). In another series, the patients with PD had larger tumor size and higher mitotic count (118). A recent meta-analysis of seven comparative studies confirmed categorically the above-mentioned findings (89).
Imatinib mesylate have played a key role as a neoadjuvant therapy in the management of GISTs (21,122). In locally advanced disease neoadjuvant imatinib may downstage the tumor to allow R0 resection or even an organ preserving intervention (11,122,136-138) despite the risk of bleeding (111). Ludvigsen et al. reported a successful PD en-block with right kidney and suprarenal gland (19). Fukuyama et al. reported even avoidance of PD after downstaging neoadjuvant therapy with imatinib (67). Recently, successful organ-preserving duodenectomies after neoadjuvant therapy in 9 of 10 cases was reported (92) similarly to other authors (82).
PD is, however, burdened by significant morbidity and longer hospital stay in comparison to local resection, probably due to the “soft” pancreas and small caliber of pancreatic duct (116,118,119). Moreover, several studies reported no significant difference in recurrence rate and disease specific survival between limited resection and pancreatoduodenectomy (55,97,101,104), which is categorically supported from the meta-analysis of Chok et al. (122) and the experience of the French Sarcoma Group (119).
R0 resection with 1–2 cm clear margin is sufficient treatment and lymph node dissection is not recommended due to the low incidence of lymphatic metastases (102). There are several options for local resection and surgical reconstruction according to the localization of the tumour. The rupture of GIST during the surgery should be avoided because is associated with nearly 100% risk for recurrence (125). Therefore, the good knowledge of anatomy, gentle handling of the tissues, and careful dissection of duodenal wall from the inferior border of pancreas, meticulous hemostasis and knowing of the possible options for duodenal reconstruction are mandatory for successful outcomes.
Laparoscopic resection is feasible and safe with reported subtotal resection with side-to-side duodenojejunostomy (100) and wedge resection (85,139). Tanaka et al. reported eighth cases successful segmental resections of duodenojejunal junction with side-to-side anastomosis comparable with 11 open procedures (140). Some authors reported laparoscopically assisted endoscopic submucosal resection of 20 mm GIST located in the third portion (57).
There are six reported cases of dGIST localized in first and second portion removed by robotic surgery: wedge resection (140-142) and segmental resection with side-to-side duodenojejunostomy (143).
Although in most cases wedge and segmental resections could be relatively straightforward, severe complications may occur such as acute pancreatitis, pancreatic fistula, significant blood loss and anastomotic stenosis (117,118). Delayed gastric emptying treating with gastrojejunostomy and the fearsome anastomotic failure necessitated secondary PD (101) and injury of the mesenteric root managed by total enterectomy have been described in the literature (132). The pitfalls in segmental resections are associated with the superior mesenteric vessels lying on the third portion, adjacent pancreas and the common blood supply, necessity to preserve ampulla Vateri (107,117).
Conclusions
dGIST is a very rare entity. It may be asymptomatic or may involve symptoms of upper GI bleeding and abdominal pain at presentation. Because of the misleading clinical presentation the differential diagnosis may be difficult. Gastrointestinal endoscopy is the most common initial diagnostic procedure while abdominal and thoracic CT scan are mandatory for accurate oncologic staging and surgical planning.
Tumours smaller than 2 cm have a low biological aggressiveness and can be followed annually by endoscopic ultrasound; the biggest tumors should undergo radical surgery (R0) (144). In contrast to the other localizations, dGIST have no uniformly adopted surgical strategy because of the low incidence, lack of experience, and complex anatomy of the duodeno-pancreatic region. Therefore, individually tailored surgical approach is recommended. R0 resection with 1–2 cm clear margin is sufficient treatment and lymph node dissection is not recommended due to the low incidence of lymphatic metastases. Tumor rupture should be avoided.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The review of literature does not need evaluation by Ethical committee and by Institutional/Regional/National Ethics committees/boards.
References
- Yang WL, Yu JR, Wu YJ, et al. Duodenal gastrointestinal stromal tumor: clinical, pathologic, immunohistochemical characteristics, and surgical prognosis. J Surg Oncol 2009;100:606-10. [Crossref] [PubMed]
- Cirocchi R, Patelli S, Castellani E, et al. Right hemicolectomy plus pancreaticoduodenectomy vs partial duodenectomy in treatment of locally advanced right colon cancer invading pancreas and/or only duodenum. Surg Oncol 2014;23:92-8. [Crossref] [PubMed]
- Hirota S, Isozaki K, Moriyama Y, et al. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science 1998;279:577-80. [Crossref] [PubMed]
- Biasco G, Velo D, Angriman I, et al. Gastrointestinal stromal tumors: report of an audit and review of the literature. Eur J Cancer Prev 2009;18:106-16. [Crossref] [PubMed]
- Miettinen M, Lasota J. Gastrointestinal stromal tumors (GISTs): definition, occurrence, pathology, differential diagnosis and molecular genetics. Pol J Pathol 2003;54:3-24. [PubMed]
- Nilsson B, Bumming P, Meis-Kindblom JM, et al. Gastrointestinal stromal tumors: the incidence, prevalence, clinical course, and prognostication in the pre-Imatinib mesylate era – a population-based study in western Sweden. Cancer 2005;103:821-9. [Crossref] [PubMed]
- Sawaki A. Rare gastrointestinal stromal tumors (GIST): omentum and retroperitoneum. Transl Gastroenterol Hepatol 2017;2:116. [Crossref] [PubMed]
- Agaimy A, Wunsch PH. Gastrointestinal stromal tumors: a regular origin in the muscularis propria, but an extremely diverse gross presentation. Langenbecks Arch Surg 2006;391:322-9. [Crossref] [PubMed]
- Miettinen M, Kopczynski J, Makhlouf HR, et al. Gastrointestinal stromal tumors, intramural leiomyomas and leiomyosarcomas in the duodenum. Am J Surg Pathol 2003;27:625-41. [Crossref] [PubMed]
- Winfield RD, Hochwald SN, Vogel SB, et al. Presentation and management of gastrointestinal stromal tumors of the duodenum. Am Surg 2006;72:719-22; discussion 722-3. [PubMed]
- Johnston FM, Kneuertz PJ, Cameron JL, et al. Presentation and management of gastrointestinal stromal tumors of the duodenum: A multi-institutional analysis. Ann Surg Oncol 2012;19:3351-60. [Crossref] [PubMed]
- Kindblom LG, Remotti HE, Aldenborg F, et al. Gastrointestinal pacemaker cell tumor (GIPACT): gastrointestinal stromal tumors show phenotypic characteristics of the interstitial cells of Cajal. Am J Pathol 1998;152:1259-69. [PubMed]
- Takahashi Y, Noguchi T, Takeno S, et al. Gastrointestinal stromal tumor of the duodenal ampulla: report of a case. Surg Today 2001;31:722-6. [Crossref] [PubMed]
- Uchida H, Sasaki A, Iwaki K, et al. An extramural gastrointestinal stromal tumor of the duodenum mimicking a pancreatic head tumor. J Hepatobiliary Pancreat Surg 2005;12:324-7. [Crossref] [PubMed]
- Hompes D, Topal B, Ectors N, et al. Gastro-intestinal stromal tumour of the duodenum: extreme presentation in two cases. Acta Chir Belg 2004;104:110-3. [Crossref] [PubMed]
- Kim SH, Kim JH, Baik GH, et al. Malignant gastrointestinal stromal tumor of the ampulla of Vater: a case report. Korean J Gastroenterol 2004;43:66-70. [PubMed]
- Sakakima Y, Inque S, Fuji T, et al. Emergency pylorus-preserving pancreatoduodenectomy followed by second-stage pancreatojejunostomy for a gastrointestinal stromal tumor of the duodenum with an intratumoral gas figure: report of a case. Surg Today 2004;34:701-5. [Crossref] [PubMed]
- Sakakura C, Hagiwara A, Soga K, et al. Long-term survival of a case with multiple liver metastases from duodenal gastrointestinal stromal tumor drastically reduced by the treatment with imatinib and hepatectomy. World J Gastroenterol 2006;12:2793-7. [Crossref] [PubMed]
- Ludvigsen L, Toxverd A, Mahdi B, et al. Successful resection of an advanced duodenal gastrointestinal stromal tumor after down-staging with Imatinib: report of a case. Surg Today 2007;37:1105-9. [Crossref] [PubMed]
- Fiore M, Palassini E, Fumagalli E, et al. Preoperative imatinib mesylate for unresectable or locally advanced primary gastrointestinal stromal tumors (GIST). Eur J Surg Oncol 2009;35:739-45. [Crossref] [PubMed]
- Hecker A, Hecker B, Bassaly B, et al. Dramatic regression and bleeding of a duodenal GIST during preoperative imatinib therapy: case report and review. World J Surg Oncol 2010;8:47. [Crossref] [PubMed]
- Frampton AE, Bong JJ, Kyriakides C, et al. En bloc resection of the pancreatic head and second part of duodenum for a duodenal gastrointestinal stromal tumor: a multi-media report. JOP 2010;11:396-400. [PubMed]
- Wall ML, Ghallab MA, Farmer M, et al. Gastrointestinal stromal tumor presenting with duodenal-jejunal intussusception: a case report. Ann R Coll Surg Engl 2010;92:e32-4. [Crossref] [PubMed]
- Machado NO, Chopra PJ, Al-Haddabi IH, et al. Large duodenal gastrointestinal stromal tumor presenting with acute bleeding managed by a Whipple resection. A review of surgical options and the prognostic indicators of outcome. JOP 2011;12:194-9. [PubMed]
- Morcos B, Al-Ahmad F. A large gastrointestinal stromal tumor of the duodenum: a case report. J Med Case Rep 2011;5:457. [Crossref] [PubMed]
- Singh S, Paul S, Khandelwal P, et al. Duodenal GIST presenting as large pancreatic head mass: an uncommon presentation. JOP 2012;13:696-9. [PubMed]
- Koontz MZ, Visser BM, Kunz PL. Neoadjuvant imatinib for borderline resectable GIST. J Natl Compr Canc Netw 2012;10:1477-82. [Crossref] [PubMed]
- Blandamura S, Alessandrini L, Bertorelle R, et al. Multiple sporadic gastrointestinal stromal tumors concomitant with ampullary adenocarcinoma: a case report with KIT and PDGFRA mutational analysis and miR-221/222 expression profile. Pathol Res Pract 2014;210:392-6. [Crossref] [PubMed]
- Bormann F, Wild W, Aksoy H, et al. A Pancreatic Head Tumor Arising as a Duodenal GIST: A Case Report and Review of the Literature. Case Rep Med 2014;2014. [Crossref] [PubMed]
- Kobayashi M, Hirata N, Nakaji S, et al. Gastrointestinal stromal tumor of the ampulla of Vater. World J Gastroenterol 2014;20:4817-21. [Crossref] [PubMed]
- Parisi A, Desiderio J, Trastulli S, et al. Robotic pancreaticoduodenectomy in a case of duodenal gastrointestinal stromal tumor. World J Surg Oncol 2014;12:372. [Crossref] [PubMed]
- Bhambare MR, Pandya JS, Waghmare SB, et al. Gastrointestinal stromal tumor presenting as palpable mass: a rare entity. World J Gastrointest Surg 2015;7:98-101. [Crossref] [PubMed]
- Okasha HH, Amin KM, Al-Shazli M, et al. A duodenal gastrointestinal stromal tumor with a large central area of fluid and gas due to fistulization into the duodenal lumen, mimicking a large duodenal diverticulum. Endosc. Ultrasound 2015;4:253-6. [Crossref] [PubMed]
- Niikura R, Serizawa T, Yamada A, et al. Safety of Regular-Dose Imatinib Therapy in Patients with Gastrointestinal Stromal Tumors Undergoing Dialysis. Case Rep Gastroenterol 2016;10:17-23. [Crossref] [PubMed]
- Yamamoto R, Kato S, Maru T, et al. The coexistence of somatostatinoma and gastrointestinal stromal tumor in the duodenum of a patient with von Recklinghausen‘s disease. Intern Med 2016;55:617-22. [Crossref] [PubMed]
- Tornambe A, Tornambe G. Duodenal gastrointestinal stromal tumor. A case report. Ann Ital Chir. 2017.6. [PubMed]
- Sawaki A, Ohashi K, Yamao K, et al. Effect of a tyrosine kinase inhibitor STI571 in a patient with hepatic metastases from a duodenal gastrointestinal stromal tumor. J Gastroenterol 2003;38:690-4. [Crossref] [PubMed]
- Sakamoto Y, Yamamoto J, Takahashi H, et al. Segmental resection of the third portion of the duodenum for a gastrointestinal stromal tumor: a case report. Jpn J Clin Oncol 2003;33:364-6. [Crossref] [PubMed]
- Kurihara N, Kikuchi K, Tanabe M, et al. Partial resection of the second portion of the duodenum for gastrointestinal stromal tumor after effective transarterial embolization. Int J Clin Oncol 2005;10:433-7. [Crossref] [PubMed]
- Goh BK, Chow PKH, Ong H, et al. Gastrointestinal stromal tumor involving the second and third portion of the duodenum: treatment by partial duodenectomy and Roux-en-Y duodenectomy. J Surg Oncol 2005;91:273-5. [Crossref] [PubMed]
- Cavallini M, Cecera A, Ciardi A, et al. Small periampullary duodenal gastrointestinal stromal tumor treated by local excision: report of a case. Tumori 2005;91:264-6. [Crossref] [PubMed]
- Vu HA, Xinh PT, Kikushima M, et al. A recurrent duodenal gastrointestinal stromal tumor with a frameshift mutation resulting in a stop codon in KIT exon 13. Genes Chromosomes Cancer 2005;42:179-83. [Crossref] [PubMed]
- Towu E, Stanton M. Gastrointestinal stromal tumor presenting with severe bleeding: a review of the molecular biology. Pediatr Surg Int 2006;22:462-4. [Crossref] [PubMed]
- Kwon SH, Cha HJ, Jung SW, et al. A gastrointestinal stromal tumor of the duodenum masquerading as a pancreatic head tumor. World J Gastroenterol 2007;13:3396-9. [Crossref] [PubMed]
- Graham J, Debiec-Rychter M, Corless CL, et al. Imatinib in the management of multiple gastrointestinal stromal tumors associated with a germline KIT K642E mutation. Arch Pathol Lab Med 2007;131:1393-6. [PubMed]
- Gupta N, Schrimer BD, Mishra R, et al. Malignant GIST masquerading as a bleeding duodenal diverticulum. Endoscopy 2007;39:E142-3. [Crossref] [PubMed]
- Mohiuddin K, Nizami S, Munir A, et al. Metastatic duodenal GIST: role of surgery combined with imatinib mesylate. Int Semin Surg Oncol 2007;4:9. [Crossref] [PubMed]
- Fernández Salazar LI, Alvarez Gago T, Sanz Rubiales A, et al. Gastrointestinal stromal tumors (GISTs): clinical aspects. Rev Esp Enferm Dig 2007;99:19-24. [Crossref] [PubMed]
- Mennigen R, Wolters HH, Schule B, et al. Segmental resection of the duodenum for gastrointestinal stromal tumor (GIST). World J Surg Oncol 2008;6:105. [Crossref] [PubMed]
- Takahashi Y, Shimizu S, Sakurai S, et al. Gastrointestinal stromal tumor in the duodenum exhibiting hemangiopericytoma-like histological pattern. Pathol Int 2009;59:98-101. [Crossref] [PubMed]
- Takeuchi H, Matsumoto T, Kusumoto T, et al. Duodenal Gastrointestinal Stromal Tumor Treated by Wedge Resection in a Patient with Neurofibromatosis Type 1: Report of a Case and Review of the Japanese Literature. Case Rep Gastroenterol 2009;3:343-9. [Crossref] [PubMed]
- Seçkin Y, Koyuncu A, Yönem O, et al. Duodenal stromal tumor: a rare and overlooked cause of massive gastrointestinal bleeding. Turk J Gastroenterol 2009;20:150-1. [PubMed]
- Hirashima K, Takamori H, Hirota M, et al. Multiple gastrointestinal tumors in neurofibromatosis type 1: report of a case. Surg Today 2009;39:979-83. [Crossref] [PubMed]
- Mehta C, Gumaste VV, Leytin A, et al. An unusual cause of upper gastrointestinal bleeding: duodenal GIST. A case report and literature review. Acta Gastroenterol Belg 2011;74:347-51. [PubMed]
- Chung JC, Kim HC, Chu CW. Segmental resection with duodenojejunostomy of gastrointestinal stromal tumor involving the duodenum. J Kor Surg Soc 2011;80:12-6. [Crossref]
- Cameron S, Schaefer IM, Schwoerer H, et al. Ten Years of Treatment with 400 mg Imatinib per Day in a Case of Advanced Gastrointestinal Stromal Tumor. Case Rep Oncol 2011;4:505-11. [Crossref] [PubMed]
- Kato M, Nakajima K, Nishida T, et al. Local resection by combined laparoendoscopic surgery for duodenal gastrointestinal tumor. Diagn Ther Endosc 2011;2011. [Crossref] [PubMed]
- Chen J, Gundara JS, Haddad R, et al. Clinicopathological and molecular aspects of foregut gastrointestinal tumors. ANZ J Surg 2014;84:52-8. [Crossref] [PubMed]
- El-Gendi A, El-Gendi S, El-Gendi M. Feasibility and oncological outcomes of limited duodenal resection in patients with primary nonmetastatic duodenal GIST. J Gastrointest Surg 2012;16:2197-202. [Crossref] [PubMed]
- Acar F, Sahin M, Ugras S, et al. A gastrointestinal stromal tumor of the third portion of the duodenum treated by wedge resection: a case report. World J Gastrointest Surg 2013;5:332-6. [Crossref] [PubMed]
- Mouaqit O, Chbani L, Maazaz K, et al. A large gastrointestinal stromal tumor of the duodenum treated by partial duodenectomy with Roux-en-Y duodenojejunostomy: a case report. J Med Case Rep 2013;7:184. [Crossref] [PubMed]
- Mokhtare M, Taghvaei T, Fakheri HT. Acute bleeding in duodenal gastrointestinal stromal tumor. Middle East J Dig Dis 2013;5:47-51. [PubMed]
- Shaw A, Jeffery J, Dias L, et al. Duodenal wedge resection for large gastrointestinal stromal tumor presenting with life-threatening haemorrhage. Case Rep Gastrointest Med 2013;2013. [Crossref] [PubMed]
- Ueda K, Hijioka M, Lee L, et al. A syncronous neuroendocrine tumor and duodenal gastrointestinal stromal tumor. Intern Med 2014;53:2483-8. [Crossref] [PubMed]
- Hankiewicz-Ziołkowska K, Soboń M, Szylberg T, et al. Duodenal bulb tumour of unknown origin. Prz Gastroenterol 2014;9:365-70. [Crossref] [PubMed]
- Manxhuka-Kerliu S, Sahatciu-Meka V, Kerliu I, et al. Small intestine gastrointestinal stromal tumor in a young adult woman: a case report and review of the literature. J Med Case Rep 2014;8:321. [Crossref] [PubMed]
- Fukuyama K, Fujikawa T, Kuramitsu S, et al. Successful treatment of bleeding large duodenal gastrointestinal stromal tumor in a patient under dual antiplatelet therapy after recent drug-eluting coronary stent implantation. BMJ Case Rep 2014;2014. [Crossref] [PubMed]
- Borgaonkar V, Deshpande S, Borgaonkar V, et al. Gastrointestinal Stromal Tumor-Single-Center Experience with Review of the Literature. Indian J Surg 2015;77:678-81. [Crossref] [PubMed]
- Jones DH, Caracciology JT, Hodul PJ, et al. Familial gastrointestinal stromal tumor syndrome: report of 2 cases with KIT Exon 11 mutation. Cancer Control 2015;22:102-8. [Crossref] [PubMed]
- Mrak K, Liegl-Atzwanger B, Haybaeck J, et al. Surgical Management of Duodenal Gastrointestinal Stromal Tumors: A Case Report. Anticancer Res 2015;35:6321-4. [PubMed]
- Kumar A, Jakhmola CK, Chauhan SS, et al. Atypical presentation of gastrointestinal stromal tumor masquerading as a large duodenal cyst: a case report. Int J Surg Case Rep 2015;9:123-6. [Crossref] [PubMed]
- Chung JC, Kim HC, Hur SM. Limited resections for duodenal gastrointestinal stromal tumors and their oncologic outcomes. Surg Today 2016;46:110-6. [Crossref] [PubMed]
- Graziosi L, Marino E, Ludovini V, et al. Unique case of sporadic multiple gastro intestinal stromal tumour. Int J Surg Case Rep 2015;9:98-100. [Crossref] [PubMed]
- Boselli C, Cirocchi R, Gemini A, et al. Urgency surgical treatment for duodenal GISTs: analysis of aged patients and review of the literature. Aging Clin Exp Res 2017;29:1-6. [Crossref] [PubMed]
- Caruso F, Nencioni M, Zefelippo A, et al. Is duodenojejunal anastomosis to the left of the superior mesenteric vessels a feasible option for tumors of the angle of Treitz. Tumori 2016;102:71-3. [Crossref] [PubMed]
- Jones JD, Oh S, Clark C, Pawa R. A Bleeding Duodenal GIST Masquerading as Refractory Peptic Ulcer Disease. ACG Case Rep J 2016;3. [Crossref] [PubMed]
- Turculeţ C, Ene D, Georgescu TF, et al. A rare case of upper digestive hemorrhage due to bleeding duodenal tumor. Chirurgia (Bucur) 2016;111:505-8. [Crossref] [PubMed]
- Huo X, Wei J, Liu X, et al. Brunner‘s gland cyst in combination with gastrointestinal stromal tumor: a case report. Oncology Letters 2016;11:3409-12. [Crossref] [PubMed]
- Mori N, Ichikawa T, Hashimoto J, et al. Cholangiolocellular Carcinoma of the Liver Exhibiting High F-18 FDG Uptake. Tokai. J Exp Clin Med 2016;41:60-4. [PubMed]
- Valli PV, Valli C, Pfammatter T, et al. Life-threatening bleeding of a duodenal gastrointestinal stromal tumor in a teenager: a rare case report. Endosc Int Open 2016;4:E1244-6. [Crossref] [PubMed]
- Crocetti D, Sapienza P, Cisano C, et al. Pancreas preserving surgery for duodenal gastrointestinal stromal tumor removal. Minerva Chirurgica 2016;71:281-5. [PubMed]
- Thillai M, Alexander N, Paramasivam S, et al. Malignant duodenal GIST in a patient with situs inversus totalis – a rare association and brief review of literature. J Gastrointest Cancer 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Elston MS, Sehgal S, Dray M, et al. A Duodenal SDH-Deficient Gastrointestinal Stromal Tumor in a Patient With a Germline SDHB Mutation. J Clin Endocrinol Metab 2017;102:1447-50. [Crossref] [PubMed]
- Vasile D, Iancu G, Iancu RC, et al. Duodenal gastrointestinal stromal tumor presenting as pancreatic head mass – a case report. Rom J Morphol Embryol 2017;58:255-9. [PubMed]
- Zioni T, Dizengof V, Kirshtein B. Laparoscopic resection of duodenal gastrointestinal stromal tumour. J Minim Access Surg 2017;13:157-60. [PubMed]
- Perfetti V, Laurini E, Aulić S, et al. Molecular and functional characterization of a new 3' end KIT juxtamembrane deletion in a duodenal GIST treated with neoadjuvant Imatinib. Oncotarget 2017;8:56158-67. [Crossref] [PubMed]
- Hakozaki Y, Sameshima S, Tatsuoka T, et al. Rectal carcinoma and multiple gastrointestinal tumors (GIST) of the small intestine in a patient with neurofibromatosis type 1: a case report. World J Surg Oncol 2017;15:160. [Crossref] [PubMed]
- Relles D, Baek J, Witkiewicz A, et al. Periampullary and duodenal neoplasms in neurofibromatosis type 1: two cases and an updated 20-year review of the literature yielding 76 cases. J Gastrointest Surg 2010;14:1052-61. [Crossref] [PubMed]
- Tien YW, Lee CY, Huang CC, et al. Surgery for gastrointestinal stromal tumors of the duodenum. Ann Surg Oncol 2010;17:109-14. [Crossref] [PubMed]
- Miki Y, Kurokawa Y, Hirao M, et al. Survival analysis of patients with duodenal gastrointestinal stromal tumors. J Clin Gastroenterol 2010;44:97-101. [Crossref] [PubMed]
- Yagishita A, Matsubayashi H, Kakushima N, et al. Gastrointestinal tumors of the duodenum: report of four cases. Clin J Gastroenterol 2011;4:162-6. [Crossref] [PubMed]
- Liang X, Yu H, Zhu LH, et al. Gastrointestinal stromal tumors of the duodenum: surgical management and survival results. World J Gastroenterol 2013;19:6000-10. [Crossref] [PubMed]
- Hoeppner J, Kuleman B, Marjanovic G, et al. Limited resection for duodenal gastrointestinal stromal tumors: surgical management and clinical outcome. World J Gastrointest Surg 2013;5:16-21. [Crossref] [PubMed]
- Yang F, Jin C, Du Z, et al. Duodenal gastrointestinal stromal tumor: clinicopathological characteristics, surgical outcomes, long term survival and predictors for adverse outcomes. Am J Surg 2013;206:360-7. [Crossref] [PubMed]
- Zhou B, Zhang M, Wu J, et al. Pancreatoduodenectomy versus local resections in the treatment of gastrointestinal tumors of the duodenum. World J Surg Oncol 2013;11:196. [Crossref] [PubMed]
- Duffaud F, Meeus P, Bachet JB, et al. Conservative vs. duodenopancreatectomy in primary duodenal gastrointestinal stromal tumors (GIST): a retrospective review of 114 patients from the French Sarcoma Group (FSG). Eur J Surg Oncol 2014;40:1369-75. [Crossref] [PubMed]
- Uçar AD, Oymaci E, Carti EB, et al. Characteristics of emergency gastrointestinal stromal tumor (GIST). Hepatogastroenterology 2015;62:635-40. [PubMed]
- Lv A, Qian H, Qiu H, et al. Organ-preserving surgery for locally advanced duodenal gastrointestinal tumor after neoadjuvant treatment. Biosci Trends 2017;11:483-9. [Crossref] [PubMed]
- Beham A, Schaefer IM, Cameron S, et al. Duodenal GIST: a single center experience. Int J Colorectal Dis 2013;28:581-90. [Crossref] [PubMed]
- Corcione F, Pirozzi F, Sciuto A, et al. Laparoscopic pancreas-preserving subtotal duodenectomy for gastrointestinal tumor. Minim Invasive Ther Allied Technol 2013;22:187-90. [Crossref] [PubMed]
- Sugase T, Takahashi T, Nakajima K, et al. Clinicopathological characteristics, surgery and survival outcomes of patients with duodenal gastrointestinal stromal tumors. Digestion 2016;94:30-6. [Crossref] [PubMed]
- Joo MK, Park JJ, Kim H, et al. Endoscopic versus surgical resection of GI stromal tumors in the upper GI tract. Gastrointest Endosc 2016;83:318-26. [Crossref] [PubMed]
- Salvi PF, Lorenzon L, Caterino S, et al. Gastrointestinal stromal tumor associated with neurofibromatosis 1: a single center experience and systematic review of the literature including 252 cases. Int J Surg Oncol 2013;2013. [Crossref] [PubMed]
- Sapalidis K, Panteli N, Strati TM, et al. Surgical management of gastrointestinal stromal tumors: a single centre's experience. Hippokratia 2015;19:73-5. [PubMed]
- Millonig G, Giesel FL, Reimann FM, et al. Gastrointestinal stromal tumor in a patient with undiagnosed neurofibromatosis type 1: an uncommon cause of extrahepatic cholestasis. Z Gastroenterol 2010;48:479-81. [Crossref] [PubMed]
- Shen C, Chen H, Yin Y, et al. Duodenal gastrointestinal stromal tumors: clinicopathological characteristics, surgery, and long-term outcome. BMC Surg 2015;15:98. [Crossref] [PubMed]
- Cirocchi R, Kelly MD, Griffiths EA, et al. A systematic review of the management and outcome of ERCP related duodenal perforations using a standardized classification system. Surgeon 2017;15:379-87. [Crossref] [PubMed]
- Chung JC, Chu CW, Cho GS, et al. Management and outcome of gastrointestinal stromal tumors of duodenum. J Gastrointest Surg 2010;14:880-3. [Crossref] [PubMed]
- Buchs NC, Bucher P, Gervaz P, et al. Segmental duodenectomy for gastrointestinal stromal tumor of the duodenum. World J Gastroenterol 2010;16:2788-92. [Crossref] [PubMed]
- Kamath AS, Sarr MG, Nagorney DM, et al. Gastrointestinal stromal tumor of the duodenum: single center institutional experience. HPB 2012;14:774-6. [Crossref]
- Uehara K, Hasegawa H, Ogiso S, et al. Gastrointestinal stromal tumor of the duodenum: diagnosis and treatment. Geka 2001;63:1058-61.
- Săftoiu A, Vilmann P, Hassan H. Utility of colour doppler endoscopic ultrasound evaluation and guided therapy of submucosal tumours of the upper gastrointestinal tract. Ultraschall Med 2005;26:487-95. [Crossref] [PubMed]
- DeWitt J, Emerson RE, Sherman S, et al. Endoscopic ultrasound-guided Trucut biopsy of gastrointestinal mesenchymal tumor. Surg Endosc 2011;25:2192-202. [Crossref] [PubMed]
- Matsui T, Mitsui H, Sekigawa K, et al. A case of a duodenal gastrointestinal stromal tumor diagnosed with the aid of diffusion-weighted magnetic resonance imaging. Clin J Gastroenterol 2009;2:384-7. [Crossref] [PubMed]
- Lee SY, Goh BKP, Sadot E, et al. Surgical strategy and outcomes in duodenal gastrointestinal stromal tumor. Ann Surg Oncol 2017;24:202-10. [Crossref] [PubMed]
- Crown A, Biehl TR, Rocha FG. Local resection for duodenal gastrointestinal stromal tumors. Am J Surg 2016;211:867-70. [Crossref] [PubMed]
- García-Molina FJ, Mateo-Vallejo F, Franco-Osorio J, et al. Surgical approach for tumors of the third and fourth part of the duodenum. Distal pancreas-preserving duodenectomy. Int J Surg 2015;18:143-8. [Crossref] [PubMed]
- Lupaşcu C, Andronic D, Moldovanu R, et al. Treatment of gastrointestinal stromal tumors--initial experience. Chirurgia (Bucur) 2010;105:657-62. [PubMed]
- Colombo C, Ronellfitsch U, Yuxin Z, et al. Clinical, pathological and surgical characteristics of duodenal gastrointestinal tumor and their influence on survival: a multi-center study. Ann Surg Oncol 2012;19:3361-7. [Crossref] [PubMed]
- Rabin I, Chikman B, Lavy R, et al. Gastrointestinal stromal tumors: a 19 year experience. Isr Med Assoc J 2009;11:98-102. [PubMed]
- Sorour MA, Kassem MI, El-Hamid A, et al. Gastrointestinal stromal tumors (GIST) related emergencies. Int J Surg 2014;12:269-80. [Crossref] [PubMed]
- Chok AY, Koh YX, Ow MY, et al. A systematic review and meta-analysis comparing pancreatoduodenectomy versus limited resection for duodenal gastrointestinal stromal tumors. Ann Surg Oncol 2014;21:3429-38. [Crossref] [PubMed]
- Lin C, Chang Y, Zhang Y, et al. Small duodenal gastrointestinal stromal tumors presenting with acute bleeding misdiagnosed as hemobilia: two case reports. Oncol Lett 2012;4:1069-71. [Crossref] [PubMed]
- Hygum K, Wulff CN, Harslof T, et al. Hypercalciemia in metastatic GIST caused by systemic elevated calcitriol: a case report and review of the literature. BMC Cancer 2015;15:788. [Crossref] [PubMed]
- Goh BK, Chow PK, Kesavan S, et al. Outcome after surgical treatment of suspected gastrointestinal tumors involving duodenum. J Surg Oncol 2008;97:388-91. [Crossref] [PubMed]
- Hohenberger P, Ronellenfitsch U, Oladeji O, et al. pattern of recurrence in patients with ruptured primary gastrointestinal stromal tumour. Br J Surg 2010;97:1854-9. [Crossref] [PubMed]
- Guller U, Tarantino I, Cerny T, et al. Revisiting a dogma: similar survival of patients with small bowel and gastric GIST. A population-based propensity score SEER analysis. Gastric Cancer 2017;20:49-60. [Crossref] [PubMed]
- Chiarugi M, Galatioto C, Lippolis P, et al. Gastrointestinal stromal tumor of the duodenum in childhood: a rare case report. BMC Cancer 2007;7:79. [Crossref] [PubMed]
- Kim JY, Lee JM, Kim KW, et al. Ectopic pancreas: CT findings with amphasis on differentiation from small gastrointestinal stromal tumor and leiomyoma. Radiology 2009;252:92-100. [Crossref] [PubMed]
- Yamashita Y, Kato J, Ueda K, et al. Contrast-enhanced endoscopic ultrasonography can predict a higher malignant potential of gastrointestinal stromal tumors by visualizing large newly formed vessels. J Clin Ultrasound 2015;43:89-97. [Crossref] [PubMed]
- Hoda KM, Rodriguez SA, Faigel DO. EUS-guided sampling of suspected GI stromal tumors. Gastrointest Endosc 2009;69:1218-23. [Crossref] [PubMed]
- Yamashita S, Sakamoto Y, Saiura A, et al. Pancreas-sparing duodenectomy for gastrointestinal stromal tumor. Am J Surg 2014;207:578-83. [Crossref] [PubMed]
- Jang SK, Kim JH, Joo I, et al. Differential diagnosis of pancreatic cancer fromother solid tumours arising from the periampullary area on MDCT. Eur Radiol 2015;25:2880-8. [Crossref] [PubMed]
- Goldstein D, Tan BS, Rossleigh M, et al. Gastrointestinal stromal tumors: correlation of 18F-FDG Gamma camera-based coincidence positron emission tomography with CT for assessment of treatment response – an AGITG study. Oncology 2005;69:326-32. [Crossref] [PubMed]
- ESMO/European Sarcoma Network Working Group. Gastrointestinal stromal tumors: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2014;25 Suppl 3:iii21-6. [Crossref] [PubMed]
- Cirocchi R, Farinella E, La Mura F, et al. Efficacy of surgery and imatinib mesylate in the treatment of advanced gastrointestinal stromal tumor: a systematic review. Tumori 2010;96:392-9. [Crossref] [PubMed]
- Gold JS, van der Zwan SM, Gonen M, et al. Outcome of metastatic GIST in the era before tyrosine kinase inhibitors. Ann Surg Oncol 2007;14:134-42. [Crossref] [PubMed]
- Bourgouin S, Hornez E, Guiramand J, et al. Duodenal gastrointestinal stromal tumors (GISTs): arguments for conservative surgery. J Gastrointest Surg 2013;17:482-7. [Crossref] [PubMed]
- Abe N, Hashimoto Y, Takeuchi H, et al. Laparoscopy-assisted full-thickness resection of the duodenum for patients with gastrointestinal stromal tumor with ulceration. Asian J Endosc Surg 2017;10:388-93. [Crossref] [PubMed]
- Xie YB, Liu H, Cui L, et al. Tumors of the angle of Treitz: a single-center experience. World J Gastroenterol 2014;20:3628-34. [Crossref] [PubMed]
- Tanaka E, Kim M, Lim JS, et al. Usefulness of laparoscopic side-to-side duodenojejunostomy for gastrointestinal stromal tumors located at the duodenojejunal junction. J Gastrointest Surg 2015;19:313-8. [Crossref] [PubMed]
- Noshiro H, Nomura A, Akashi M, et al. Pure robotic surgery for intraluminally growing gastrointestinal stromal tumors around the esophagogastric junction or pyloric ring. Hepatogastroenterology 2015;62:629-34. [PubMed]
- Downs-Canner S, Van de Vliet WJ, Tholen SJJ, et al. Robotic surgery for benign duodenal tumors. J Gastrointest Surg 2015;19:306-12. [Crossref] [PubMed]
- Huscher CG, Mingoli A, Sgarzini G, et al. Transoral extraction of a laparoscopically resected large gastric GIST. J Laparoendosc Adv Surg Tech A 2013;23:707-9. [Crossref] [PubMed]
Cite this article as: Popivanov G, Tabakov M, Mantese G, Cirocchi R, Piccinini I, D’Andrea V, Covarelli P, Boselli C, Barberini F, Tabola R, Pietro U, Cavaliere D. Surgical treatment of gastrointestinal stromal tumors of the duodenum: a literature review. Transl Gastroenterol Hepatol 2018;3:71.