Precision care for Barrett’s esophagus
Introduction
Modern recognition and management of Barrett’s esophagus (BE), a lesion that predisposes to esophageal adenocarcinoma (EAC), depends on diagnostic accuracy, risk assessment, technical expertise and consideration of many options to best tailor therapy for every patient with the disease. Since gastroesophageal reflux disease (GERD) is also present and plays a key role in disease biological behavior, its concomitant management is an essential element in therapy. Proton pump inhibitor (PPI)-induced reduction in esophageal acid exposure is thought to prevent cellular changes leading to dysplasia and cancer in BE (1,2). The use of ablation and/or resection has been shown to favorably affect the evolution of Barrett’s dysplasia towards EAC. New tools for early diagnosis of dysplasia in BE and its surveillance over time promise to facilitate monitoring of the disease over time.
Precision or personalized medicine (PM) is a recently introduced concept that customizes medical decisions, treatments, practices, or products to individual patients. In this healthcare model, the use of diagnostic testing helps select optimal therapies based on a patient’s genetic or other molecular, cellular or clinical assessments. Tools employed in precision medicine may include imaging, molecular diagnostics, as well as clinical analytics. BE is a relevant prototype for implementation of PM that aims to cost- and comparatively-effective therapies ultimately aiming at EAC elimination. In recent years, many developments have allowed differential management of patients with BE based on PM principles.
As outlined in Figure 1, this overview provides the current evidence for an optimally tailored PM therapeutic approach to BE; it can be divided in four major elements, demographic, biomolecular (disease phenotypic), clinical (endoscopic or surgical) and patient-driven precision care. These elements are individually addressed below. All these elements should be considered important in the overall decision-making for any given patient with BE. For example, a short-segment, non-dysplastic BE without associated hiatal hernia in a 45-year asymptomatic female without unfavorable biology will require less intense management than a long-segment, dysplastic and nodular BE in the presence of a large sliding hiatal hernia in a 50-year-old obese male with heartburn and regurgitation despite PPI therapy.
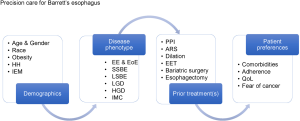
Demographic precision care
Age and gender
BE is an acquired condition that is often discovered during endoscopy in middle-aged or older adults, but it may also be seen in children. The prevalence of BE in the general population ranges from 0.4–20% with a male to female ratio of approximately 2:1 (3). Based on an epidemiological study that showed a 1.6% prevalence of BE in the Swedish general population, one can estimate 3.3 million individuals with BE living in the US (4). Among patients undergoing endoscopy for chronic GERD symptoms, long-segment BE can be found in 3–5%, whereas 10–15% have short-segment BE. In a US study, the prevalence of BE (mostly short-segment) was 6.8% among 961 patients undergoing a colonoscopy, 5.6% among those who never had heartburn and 8.3% among those with history of heartburn (5). Screening programs based upon reflux symptoms alone are inadequate to identify patients with BE since 44% of patients with BE report no heartburn or regurgitation within 3 months of the diagnosis. The overall sensitivity of endoscopy and biopsy for detection of BE is approximately 80% but it varies with the length of involved mucosa, with detection being higher in those with long-segment BE. The overall incidence of EAC is low, but it increases with age and it is higher for men without GERD than for women with GERD at any age (6). The efficacy of endoscopic ablation therapy (EET) does not differ by sex or race (7).
Race
BE is uncommon in blacks. Some studies show a similar prevalence in Hispanics to that in Caucasians and others show a lesser prevalence. BE is less prevalent in Asian countries, with a pooled prevalence of BE 1.3 percent, of which 82% short-segment BE. In the US radiofrequency ablation (RFA) patient registry, women had shorter BE and less-aggressive histology. Although the tendency toward BE in men was absent in blacks and Asians, post-RFA stricture formation was more common among women and Asians (7).
Obesity
A well-established risk factor for GERD, obesity is also a risk factor for BE and EAC. Increased body mass index (BMI) and waist-to-hip ratio (WHR) have been associated with BE. A meta-analysis of 11 observational studies showed a small increase in BE risk (OR 1.4; 95% CI, 1.1–1.6) in patients with a BMI >30 kg/m2. Abdominal obesity as measured by a high waist to hip ratio (≥0.9 in males and ≥0.85 in females) is associated with an increase in both GERD and BE risk. In a pilot case-control study of consecutive Caucasian men with BE versus controls who had GERD without BE, the abdominal diameter index (ADI, sagittal abdominal diameter divided by thigh circumference) was a more powerful predictor of the presence of BE than BMI and WHR. When controlling for age, smoking, and BMI, an ADI ≥0.60 was a significant independent risk factor for BE (OR =5.7; 95% CI, 1.29–25.4) (8).
Aspirin (ASA) and nonsteroidal anti-inflammatory drugs (NSAIDs)
To what degree, if any, the use of ASA or NSAIDs, by inhibiting cyclooxygenase-2 (COX-2) expression, can decrease the risk of BE is uncertain. Although the regular use of NSAIDs is associated with a reduced risk of EAC, it is unclear if NSAID use reduces the risk of BE. In a pooled analysis of six case-control studies, regular NSAID use was not associated with the risk of BE while similar findings were seen in those on ASA or non-ASA NSAIDs. The authors suggested that the previously reported inverse association between NSAID use and EAC might be through reducing the risk of neoplastic progression in patients with BE (9). In a case-controlled study of 434 patients, current ASA—but not NSAID—use decreased the risk of BE by 44% (OR 0.56; 95% CI, 0.39–0.80). In contrast, a large, population based, case-controlled study failed to demonstrate an association with ASA use and BE but showed a 31% BE risk reduction associated with NSAIDs (OR 0.69; 95% CI, 0.49–0.97).
Family history
Either because of shared environmental exposures or inheritance, or both, there is a familial aggregation of BE (10). Germline mutations have been associated with BE and EAC. Attention to family history and proper screening and surveillance is recommended, particularly in young adults with GERD symptoms. A prediction model has been recently devised to effectively identify high-risk individuals for screening and surveillance, thereby allowing early intervention, and reducing mortality from EAC (11).
Hiatal hernia
Esophago-gastric junction (EGJ) incompetence, hiatal hernia and impaired esophageal clearance determine the likelihood of GERD symptoms, erosive esophagitis (EE), and BE. Yet, although excessive esophageal acid exposure plays a pathogenetic role in long-segment BE, there is little evidence supporting this for short-segment BE where the acid pocket, also known as intra-sphincteric reflux, may be more important (12).
A systematic review and meta-analysis of 33 studies comprising 4,390 BE patients, quantified the risk of BE associated with hiatal hernia. Even after adjusting for reflux and BMI, hiatal hernia was associated with an increased risk of BE of any length (OR 3.94; 95% CI, 3.02–5.13). The short segment BE (SSBE) subgroup showed an increased risk (OR 2.87; 95% CI, 1.75–4.70), while the strongest association was seen with long segment BE (LSBE) (OR 12.67; 95% CI, 8.33–19.25) (13).
EAC after endoscopic therapy for high-grade dysplasia (HGD) or intramucosal cancer (IMC) in BE is associated with large hiatal hernia. This was shown in a retrospective review of consecutive 223 patients with BE (HGD or IMC) who were treated by endoscopic RFA. Recurrence or new development of adenocarcinoma was found in 20 patients (11%) with median time to recurrence of 11.5 months. Hiatal hernia size ≥4 cm was an independent predictor of recurrent or metachronous adenocarcinoma (OR 3.649, P=0.0233) (14).
The presence of sliding hiatal hernia may negatively affect the efficacy of ablative therapy for Barrett’s dysplasia. Patients with larger hiatal hernias and longer BE are more likely to experience failure or nonhealing after RFA and they require more treatment sessions to achieve successful eradication of BE (15). The newly available pear-shaped cryoballoon shows promise in effectively ablation the distal esophagus when a hiatal hernia is present.
Ineffective esophageal motility (IEM)
A retrospective case-control study on GERD patients undergoing endoscopy and high-resolution esophageal manometry, was performed in 201 patients (101 GERD with BE and 100 GERD without BE) to examine the role of esophageal dysmotility classified into: IEM, fragmented peristalsis and absence of peristalsis, and lower esophageal sphincter (LES) hypotonicity. In a multivariate analysis, the presence of EMD (OR 3.99; 95% CI, 1.71–9.28; P=0.001) particularly IEM and LES hypotonicity and hiatal hernia (OR 5.60; 95% CI, 2.45–12.76; P<0.001), were independently associated factors of BE (16). IEM contributes to poor esophageal clearance and increased esophageal acid and bile exposure particularly while supine and at night, leading to BE and possibly, its progression to dysplasia.
Phenotypic precision care
EE
EE, ulcers, stricture, and upper gastrointestinal bleeding may be frequently seen in patients with long-segment BE. EE independently increases the risk for BE fivefold (17). BE prevalence is the same in patients with and without peptic strictures (18). Aggressive treatment of EE with medical and/or surgical therapy is essential because it facilitates the endoscopic recognition and histologic assessment of dysplasia and its management with endoscopic eradication therapies.
Eosinophilic esophagitis (EoE)
EoE may overlap with GERD and may be present in patients with BE and it should be recognized prior to EET since balloon-based RFA or cryotherapy may lead to esophageal perforation. In all cases, acquisition of biopsies proximal to the BE segment is important to establish the diagnosis since it may not be endoscopically recognizable.
SSBE
Patients with SSBE had a shorter history of GERD symptoms or no symptoms at all. The risk of EAC is directly related to the extent of BE. Because of the limited mucosal involvement, patients with SSBE have a lower incidence of dysplasia. In one study, the prevalence and incidence of dysplasia was significantly higher in those with long-segment (24% vs. 8%) and EAC was found only in patients with long BE. During follow-up, dysplasia developed significantly more often in patients with long-segment BE (8% vs. 4%) (19). Patients with SSBE reflux mostly have upright reflux, higher LES pressures and better contractility than those with LSBE (20).
LSBE
LSBE can be found in 3–5% of patients who undergo endoscopy for chronic GERD symptoms, in contrast to 10–15% found with SSBE. EAC risk is up to 15 times higher in patients with LSBE. In one study, a 5-cm difference in length increased the risk for EAC by 1.7-fold (21). Patients with LSBE also had more severe, bi-positional and proximal reflux. Given the higher acid exposure and cancer risk, patients with LSBE are generally managed more aggressively, with medical or surgical therapy for GERD and, in many instances, more frequent endoscopic surveillance.
Low-grade dysplasia (LGD)
The diagnosis of LGD in BE and its clinical significance is limited by the random nature of endoscopic tissue sampling and by inter-observer variation (22). Since a field effect has been described in EAC (23), detection of molecular and cellular changes abnormalities in this expanded field may overcome the limitations of random sampling in non-dysplastic or LGD, allowing for earlier diagnosis of HGD and EAC. A tissue systems pathology approach that quantifies both epithelial and stromal abnormalities may facilitate the distinction of HGD from non-dysplastic BE with reactive atypia (24). This imaging approach has also been demonstrated to predict incident progression in BE, by objectively quantifying molecular and cellular features that precede definitive morphologic changes (25). The assay employs multiplexed immunofluorescence labeling of 9 epithelial and stromal biomarkers in formalin-fixed paraffin-embedded biopsies followed by scanning and automated image software analysis.
If LGD is confirmed, patients should be considered for endoscopic eradication therapy (EET) using ablation and/or resection. A randomized trial with 136 patients with low-grade dysplasia revealed that RFA decreased the risk of progression to HGD by 25% and EAC by 7.4% as compared to no treatment. Adverse events occurred in 19.1% of patients receiving ablation, most commonly stricture formation (11.8%) that resolved by endoscopic dilation (26). Alternatively, surveillance endoscopy should be performed every 6 months for 1 year and then annually until there is reversion to non-dysplastic BE.
HGD and IMC
The management of HGD and IMC involves careful endoscopic staging using the Paris classification and histologic assessment, followed by EET or esophagectomy. EET may involve endoscopic resection (ER) alone, particularly for nodular disease, ablation using radiofrequency or cryotherapy for flat mucosal involvement, or a combination of resection and ablation (27). A thorough endoscopic inspection by an expert endoscopist using a high definition endoscope should be performed to determine if there are any visible lesions that require endoscopic resection for precise staging and successful treatment. Visible lesions, which are often slightly raised and nodular but occasionally flat, may harbor advanced histology such as submucosal invasive cancer, poorly differentiated cancer or lymphatic invasion that may change the approach towards esophagectomy. A concerning report from the Netherlands suggests that non-expert endoscopists often fail to appreciate visible lesions in BE: 76% of patients referred to a Barrett’s treatment center after “random” surveillance biopsies demonstrated dysplasia had a visible lesion on repeat endoscopy (28). After ER of visible dysplasia, ablation to eliminate the remaining metaplasia is recommended to reduce the risk of metachronous dysplasia and cancer (29). In a UK registry study of 335 patients with BE and early neoplasia, nodules were removed by ER, and patients underwent RFA every 3 months. At 12 months of follow-up, HGD was eradicated in 86%, all dysplasia in 81%, and BE in 62% of patients. Nineteen months after initial therapy, 94% of patients were dysplasia free (30).
There are two different techniques for ER: cap-assisted and band ligation ER. A randomized trial comparing the two techniques found them to be equally safe, with a 5% perforation rate in each arm. However, band ligation was significantly faster, requiring 34 min, compared to 50 min for cap-assisted ER (31). Endoscopic submucosal dissection (ESD), allows for en bloc resection of superficial HGD and IMC of any size but it requires more training and time to perform (Figure 2). A randomized trial comparing ER and ESD in patients with unifocal nodular HGD and IMC <3 cm found a higher rate of en bloc resection, a higher rate of R0 resection, and a significantly longer procedure time with ESD (32). While it is technically possible to perform a complete en bloc circumferential resection of short or LSBE using ESD and thereby completely eradicate the disease in one session, refractory strictures following this type of treatment are common and an effective stricture prevention method is needed before advocating for this type of treatment (33). Instead, combination therapy with endoscopic resection of visible lesions followed by ablation of residual flat BE is recommended because of its generally high success rate and favorable side-effect profile.

Commonly used ablation modalities for BE include RFA, cryotherapy and argon plasma coagulation. RFA uses radiofrequency energy delivered on contact with the target mucosa, resulting in water vaporization, coagulation of proteins, and tissue necrosis. Several energy-delivery systems may treat long-segment circumferential BE, or short segments and focal lesions. Cryotherapy is another ablative modality that destroys tissue with rapid freezing and thawing through the use of either liquid nitrogen or carbon dioxide, or a hand-held cryoballoon delivering nitrous oxide (34). Argon plasma coagulation can be performed in conjunction with submucosal injection of saline (hybrid APC) to reduce the potential for deep injury; it is particularly suitable for smaller areas of BE (35).
Clinical precision care
Proton pump inhibition (PPI) therapy
Acid suppression is essential in the management of patients with BE, regardless of whether or not endoscopic or surgical therapy is pursued. Two retrospective studies suggest that effective control of esophageal pH may decrease the chances for dysplasia. In a VA study, the cumulative incidence of dysplasia was significantly lower among patients on PPI than in those who received no therapy or used H2-receptor antagonists. Further, among those on PPIs, a longer duration of use was associated with less frequent occurrence of dysplasia (36). Another study found that ongoing PPI therapy appeared beneficial in the prevention of dysplasia and cancer in patients with BE and suggested that all patients, even those with no esophagitis or symptoms, should continue acid suppression in the long term (37). Hence, control of the esophageal acid (and bile) exposure by mechanical and pharmacologic means seems quite important in the pathogenesis and natural history of BE. Regarding acid control, there are two possible scenarios: First, acid control takes place before the development of BE and either aborts the formation of metaplasia or is associated with shorter segment metaplasia. Second, effective acid control occurs after the formation of metaplasia and leads to less dysplasia and cancer, a chemoprevention effect (38).
A study of 110 asymptomatic patients on PPI with a history of GERD and/or BE found that only 58% of patients with GERD and 50% with BE normalized their pH on PPI therapy (39). Since most such patients are asymptomatic, clinical assessment is not an adequate measure of acid reflux control and ambulatory pH monitoring, while on PPI therapy, is recommended. The progression of BE to dysplasia and EAC is incompletely understood, but increased and disordered proliferation is a key cellular event. In ex vivo organ culture experiments, cell proliferation is increased after exposure to short pulses of acid, while proliferation is reduced in BE specimens taken from patients with esophageal acid exposure normalized by PPI therapy. In long-term clinical studies, consistent and profound intra-esophageal acid suppression with PPI decreases cell proliferation and increases differentiation in BE, but the clinical importance of such favorable effects on these surrogate markers is not clear (40).
Ongoing pathologic acid exposure is a risk factor for persistent IM following RFA. In a multivariate multiple logistic regression analysis of 45 patients who were treated with RFA, moderate to severe esophageal acid exposure and large hiatal hernia were independent factors associated with poor eradication of metaplasia (41). In another study of 37 patients treated with RFA, uncontrolled, mostly weakly acidic reflux despite twice-daily PPI therapy before therapy, longer segment BE and sizeable hiatal hernia increased the chances for persistent BE after ablation (1). Normalization of esophageal acid exposure—albeit not formally proven in RCT studies—should be beneficial in preventing metaplasia in GERD patients and potentially diminish the likelihood of neoplastic progression of BE. In long-segment BE, acid reflux and symptom scores improve with increased PPI therapy based on pH monitoring reaching the same level as after a successful fundoplication. Such normalization of acid reflux in both groups is associated with reduced papillary length, basal cell thickness, intercellular space dilation, and chronic inflammation (42).
A nationwide case-control study in Denmark among 9883 patients with a new diagnosis of BE investigated if the intensity and adherence of PPI use affected the risk of EAC and found that the relative risk of EAC or HGD was 2.2 (0.7-6.7) and 3.4 (95% CI: 1.1-10.5) in long-term low- and high-adherence PPI users respectively. There were no cancer-protective effects from PPI use and the high-adherence and long-term use of PPI were associated with a significantly increased risk (43). Because of this, in the absence of GERD symptoms or esophagitis, the use of high-dose PPI therapy and the role of ambulatory pH monitoring in guiding such therapy are subjects of ongoing research, and there is no conclusive evidence to suggest that these strategies should be pursued (44).
Anti-reflux surgery (ARS)
At 2 years of follow-up, a comparison of post-RFA treatment with either daily PPI or laparoscopic Nissen fundoplication found recurrence of BE in 20% of the PPI group versus 9.1% of the surgical group (45). A systematic database review examined the long-term role of ARS in BE, specifically, symptoms, morbidity and surgical failures as well as rates of progression, regression and EAC. Although ARS improves patients’ GERD-specific quality of life (QoL), there is insufficient evidence to recommend it over medical therapy for cancer risk reduction and continued endoscopic surveillance is needed (46).
Endoscopic dilation
Patients with BE may have esophageal strictures in the setting of concomitant esophagitis and ulceration or as a result of prior EET. Endoscopic dilation in such patients is of paramount importance not only for relief of dysphagia but also to allow EET to be implemented or continued. Focal contact and non-balloon-based circumferential treatments are preferable in such cases.
EET
EET is effective and durable for the treatment of BE, with low rates of recurrence of dysplasia. A recent systematic review of 41 studies reported pooled incidence rates of recurrent metaplasia, dysplastic BE, and HGD/EAC after RFA of 9.5%, 2.0%, and 1.2% per patient-year, respectively. When all endoscopic modalities were included, pooled incidence rates were 7.1%, 1.3%, and 0.8% per patient-year, respectively. Increased age and length of BE segment and higher grade of dysplasia were predictive of recurrence (47). Current guidelines for surveillance following ablation are limited, with recommendations based on low-quality evidence and expert opinion. Optical coherence tomography and wide-area tissue sampling with computer-assisted analysis show promise as adjunctive surveillance modalities (44).
Bariatric surgery
Laparoscopic sleeve gastrectomy (SG) is the most frequently performed bariatric procedure worldwide but it may increase the risk of esophagitis and BE. Besides weight regain, GERD is the most common reason for conversion to Roux-en-Y gastric bypass (RYGB). In a small cohort study of SG patients who did not suffer from symptomatic reflux or hiatal hernia preoperatively and had a follow-up of >10 years, endoscopy revealed de novo hiatal hernias in 45% and BE in 15%, while 14% were converted to RYGB due to intractable reflux. These results suggest that pre-existing large hiatal hernia, GERD, and BE are relative contraindications to SG (48).
Esophagectomy
Esophagectomy is the initial therapeutic approach for those patients with deep T1bN0M0 lesions and some with clinical T2N0M0 lesions. There is increasing evidence that well-differentiated superficial submucosal invasive cancer (T1b SM1) without lymphovascular invasion has a very low risk of lymph node metastasis and is effectively treated by en bloc endoscopic resection, particularly using ESD (49). However, T1b tumors with deep submucosal invasion or lymphovascular invasion or poorly differentiated histology should be treated by esophagectomy in patients who are fit for surgery. Initial chemoradiotherapy rather than upfront esophagectomy is often suggested for patients with thoracic esophageal or esophagogastric junction tumors and full-thickness (T3) involvement of the esophagus with/without nodal disease and for selected patients with T4a disease with local invasion that can be resected en bloc, and who have no evidence of distant metastases. Recurrent BE or EAC after curative esophagectomy is not uncommon and represents metachronous disease. In one study, 50% of such patients required subsequent treatment either with repeat surgery or ablative therapy. Hence, endoscopic surveillance in patients after “curative” esophagectomy for Barrett’s dysplasia or localized cancer should always be pursued (50).
Patient-driven precision care
Comorbidities
The true impact of BE on life expectancy and the efficacy of long-term surveillance remains debatable. Endoscopic surveillance of BE looking for HGD or EAC is probably not cost-effective and many patients with BE die of causes other than EAC. In a study involving 2,067 person-years of follow-up of 640 patients, 17 progressed to HGD or EAC. Those with BE ≥2 cm had an annual incidence ratio (IR) of 1.2% and >8-fold increased relative risk of HGD or EAC, compared to BE<2 cm {IR 0.14% (incidence rate ratio (IRR) 8.6; 95% CI, 4.5–12.8)}. Limiting the surveillance cohort after the first endoscopy to patient with BE ≥2 cm, or dysplasia, may improve cost-effectiveness (51). This can be further improved if one considers various important co-morbidities, such as cardiopulmonary disease or cancer with potential impacts to the patients’ longevity.
Adherence
Adherence to World Cancer Research Fund guidelines is independent protective factor (OR 0.51; 95% CI, 0.37–0.67) of disease progression to EAC. Disease progression is associated with reduced adherence to guidelines on physical activity, sedentary habits, fruit consumption and processed meat consumption (52). Further, adherence to quality indicators and surveillance guidelines in BE is low and more than half of patients with non-dysplastic BE undergo surveillance EGD sooner than recommended (53). Adherence to an endoscopic therapeutic regimen is important for longitudinal management of BE. Patients seen in a clinical consultation prior to endoscopic therapy for BE-associated neoplasia are more likely to adhere to demonstrate treatment, compared to those referred for open-access endoscopy (54).
QoL
Health-related QoL (HRQoL) scores are significantly reduced in BE patients compared with controls from the general population but similar to those of patients with GERD. Yet, frequently BE patients have insufficient understanding of the disease, inaccurate perceptions of cancer risk, and an unnecessary psychological burden, all amenable to clarification and better guidance (55). In a Taiwanese study, 84 BE patients were compared with 168 healthy adults and were found to have significantly lower QoL scores, particularly in pain, discomfort, sleep and rest and dependence on medications or treatments but there were no significant differences in social and psychological domains (56).
Fear of cancer
Patients with BE greatly overestimate their cancer risk and are willing to accept low success rates and high risks of complications to undergo endoscopic therapy. Underlying psychological factors, particularly anxiety, may influence risk perceptions. Greater emphasis on patient-centered discussions about BE and cancer risk may be helpful for reducing patients’ psychological distress and engaging patients in shared decision-making regarding management strategies (57).
Conclusions
While there have been significant advances in detection and management of BE, numerous challenges remain in identifying patients with BE and tailoring surveillance to provide effective and efficient detection of dysplasia and early cancer. Patients should be counselled appropriately about the roles of acid suppression, the effects of medications such as PPIs and NSAIDs, and the incidence of progression to dysplasia and cancer. Knowledge of the risk factors for BE and progression to dysplasia can help tailor a PM approach with more aggressive screening and surveillance targeted at patients that are most likely to benefit. Once dysplasia is identified, endoscopic resection should be performed for visible lesions in BE and ablation should be reserved for flat areas of BE after resection of visible lesions. Early esophageal cancer, including most T1a and some superficial T1b tumors, can be treated successfully with low morbidity using endoscopic resection. Continued surveillance after endoscopic and surgical treatment is important because recurrence of BE is not uncommon.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Krishnan K, Pandolfino JE, Kahrilas PJ, et al. Increased risk for persistent intestinal metaplasia in patients with Barrett's esophagus and uncontrolled reflux exposure before radiofrequency ablation. Gastroenterology 2012;143:576-81. [Crossref] [PubMed]
- Overholt BF. Acid suppression and re-epithelialization after ablation of Barrett's esophagus. Dig Dis 2000-2001;18:232-9. [Crossref] [PubMed]
- Cook MB, Wild CP, Forman D. A systematic review and meta-analysis of the sex ratio for Barrett's esophagus, erosive reflux disease, and nonerosive reflux disease. Am J Epidemiol 2005;162:1050-61. [Crossref] [PubMed]
- Sampliner RE. A population prevalence of Barrett's esophagus--finally. Gastroenterology 2005;129:2101-3. [Crossref] [PubMed]
- Rex DK, Cummings OW, Shaw M, et al. Screening for Barrett's esophagus in colonoscopy patients with and without heartburn. Gastroenterology 2003;125:1670-7. [Crossref] [PubMed]
- Rubenstein JH, Scheiman JM, Sadeghi S, et al. Esophageal adenocarcinoma incidence in individuals with gastroesophageal reflux: synthesis and estimates from population studies. Am J Gastroenterol 2011;106:254-60. [Crossref] [PubMed]
- Pasricha S, Li N, Bulsiewicz WJ, et al. Sex and race and/or ethnicity differences in patients undergoing radiofrequency ablation for Barrett's esophagus: results from the U.S. RFA Registry. Gastrointest Endosc 2015;82:276-84. [Crossref] [PubMed]
- Baik D, Sheng J, Schlaffer K, et al. Abdominal diameter index is a stronger predictor of prevalent Barrett's esophagus than BMI or waist-to-hip ratio. Dis Esophagus 2017;30:1-6. [Crossref] [PubMed]
- Thrift AP, Anderson LA, Murray LJ, et al. Nonsteroidal Anti-Inflammatory Drug Use is Not Associated With Reduced Risk of Barrett's Esophagus. Am J Gastroenterol 2016;111:1528-35. [Crossref] [PubMed]
- Juhasz A, Mittal SK, Lee TH, et al. Prevalence of Barrett’s Esophagus in first degree relatives of patients with esophageal adenocarcinoma. J Clin Gastroenterol 2011;45:867-71. [Crossref] [PubMed]
- Sun X, Elston RC, Barnholtz-Sloan JS, et al. Predicting Barrett's Esophagus in Families: An Esophagus Translational Research Network (BETRNet) Model Fitting Clinical Data to a Familial Paradigm. Cancer Epidemiol Biomarkers Prev 2016;25:727-35. [Crossref] [PubMed]
- Roman S, Kahrilas PJ. Mechanisms of Barrett's oesophagus (clinical): LOS dysfunction, hiatal hernia, peristaltic defects. Best Pract Res Clin Gastroenterol 2015;29:17-28. [Crossref] [PubMed]
- Andrici J, Tio M, Cox MR, et al. Hiatal hernia and the risk of Barrett's esophagus. J Gastroenterol Hepatol 2013;28:415-31. [Crossref] [PubMed]
- Yasuda K, Choi SE, Nishioka NS, et al. Incidence and predictors of adenocarcinoma following endoscopic ablation of Barrett's esophagus. Dig Dis Sci 2014;59:1560-6. [Crossref] [PubMed]
- Korst RJ, Santana-Joseph S, Rutledge JR, et al. Effect of hiatal hernia size and columnar segment length on the success of radiofrequencyablation for Barrett's esophagus: a single-center, phase II clinical trial. J Thorac Cardiovasc Surg 2011;142:1168-73. [Crossref] [PubMed]
- Bazin C, Benezech A, Alessandrini M, et al. Esophageal Motor Disorders Are a Strong and Independent Associated Factor of Barrett's Esophagus. J Neurogastroenterol Motil 2018;24:216-25. [Crossref] [PubMed]
- Ronkainen J, Talley NJ, Storskrubb T, et al. Erosive esophagitis is a risk factor for Barrett's esophagus: a community-based endoscopic follow-up study. Am J Gastroenterol 2011;106:1946-52. [Crossref] [PubMed]
- Kim SL, Wo JM, Hunter JG, et al. The prevalence of intestinal metaplasia in patients with and without peptic strictures. Am J Gastroenterol 1998;93:53-5. [Crossref] [PubMed]
- Sharma P, Morales TG, Bhattacharyya A, et al. Dysplasia in short-segment Barrett's esophagus: a prospective 3-year follow-up. Am J Gastroenterol 1997;92:2012-6. [PubMed]
- Loughney T, Maydonovitch CL, Wong RK. Esophageal manometry and ambulatory 24-hour pH monitoring in patients with short and long segment Barrett's esophagus. Am J Gastroenterol 1998;93:916-9. [PubMed]
- Rudolph RE, Vaughan TL, Storer BE, et al. Effect of segment length on risk for neoplastic progression in patients with Barrett esophagus. Ann Intern Med 2000;132:612-20. [Crossref] [PubMed]
- Montgomery E, Bronner MP, Goldblum JR, et al. Reproducibility of the diagnosis of dysplasia in Barrett esophagus: a reaffirmation. Hum Pathol 2001;32:368-78. [Crossref] [PubMed]
- Prevo LJ, Sanchez CA, Galipeau PC, et al. p53-mutant clones and field effects in Barrett's esophagus. Cancer Res 1999;59:4784-7. [PubMed]
- Prichard JW, Davison JM, Campbell BB, et al. TissueCypher: A Systems Biology Approach to Anatomic Pathology. J Pathol Inform 2015;6:48. [Crossref] [PubMed]
- Critchley-Thorne RJ, Duits LC, Prichard JW, et al. A Tissue Systems Pathology Assay for High-Risk Barrett's Esophagus. Cancer Epidemiol Biomarkers Prev 2016;25:958-68. [Crossref] [PubMed]
- Phoa KN, van Vilsteren FG, Weusten BL, et al. Radiofrequency ablation vs endoscopic surveillance for patients with Barrett esophagus and low-grade dysplasia: a randomized clinical trial. JAMA 2014;311:1209-17. [Crossref] [PubMed]
- Subramanian CR, Triadafilopoulos G. Endoscopic treatments for dysplastic Barrett's esophagus: resection, ablation, what else? World J Surg 2015;39:597-605. [Crossref] [PubMed]
- Schölvinck DW, van der Meulen K, Bergman JJGHM, et al. Detection of lesions in dysplastic Barrett's esophagus by community and expert endoscopists. Endoscopy 2017;49:113-120. [PubMed]
- Gaddam S, Wani S. Endoscopic therapy of Barrett esophagus. Gastrointest Endosc Clin N Am 2013;23:1-16. [Crossref] [PubMed]
- Haidry RJ, Dunn JM, Butt MA, et al. Radiofrequency ablation and endoscopic mucosal resection for dysplastic Barrett’s esophagus and early esophageal adenocarcinoma: outcomes of the UK National Halo RFA Registry. Gastroenterology 2013;145:87-95. [Crossref] [PubMed]
- Pouw RE, van Vilsteren FG, Peters FP, et al. Randomized trial on endoscopic resection-cap versus multiband mucosectomy for piecemeal endoscopic resection of early Barrett's neoplasia. Gastrointest Endosc 2011;74:35-43. [Crossref] [PubMed]
- Terheggen G, Horn EM, Vieth M, et al. A randomised trial of endoscopic submucosal dissection versus endoscopic mucosal resection for early Barrett's neoplasia. Gut 2017;66:783-93. [Crossref] [PubMed]
- Jain D, Singhal S. Esophageal Stricture Prevention after Endoscopic Submucosal Dissection. Clin Endosc 2016;49:241-56. [Crossref] [PubMed]
- Canto MI, Shaheen NJ, Almario JA, et al. Multifocal nitrous oxide cryoballoon ablation with or without EMR for treatment of neoplastic Barrett's esophagus (with video). Gastrointest Endosc 2018;88:438-446.e2. [Crossref] [PubMed]
- Pech O. Hybrid Argon Plasma Coagulation in Patients With Barrett Esophagus. Gastroenterol Hepatol (N Y) 2017;13:610-12. [PubMed]
- El‐Serag HB, Aguirre TV, Davis S, et al. Proton pump inhibitors are associated with reduced incidence of dysplasia in Barrett's esophagus. Am J Gastroenterol 2004;99:1877-83. [Crossref] [PubMed]
- Hillman LC, Chiragakis L, Shadbolt B, et al. Proton-pump inhibitor therapy and the development of dysplasia in patients with Barrett's oesophagus. Med J Aust 2004;180:387-91. [PubMed]
- Triadafilopoulos G. Proton pump inhibitors in Barrett's esophagus: Pluripotent but controversial. Eur Surg 2008;40:58-65. [Crossref]
- Gerson LB, Boparai V, Ullah N, et al. Oesophageal and gastric pH profiles in patients with gastro-oesophageal reflux disease and Barrett's oesophagus treated with proton pump inhibitors. Aliment Pharmacol Ther 2004;20:637-43. [Crossref] [PubMed]
- Fitzgerald RC, Lascar R, Triadafilopoulos G. Review article: Barrett's oesophagus, dysplasia and pharmacologic acid suppression. Aliment Pharmacol Ther 2001;15:269-76. [Crossref] [PubMed]
- Akiyama J, Marcus SN, Triadafilopoulos G. Effective intra-esophageal acid control is associated with improved radiofrequency ablation outcomes in Barrett's esophagus. Dig Dis Sci 2012;57:2625-32. [Crossref] [PubMed]
- Baldaque-Silva F, Vieth M, Debel M, et al. Impact of gastroesophageal reflux control through tailored proton pump inhibition therapy or fundoplication in patients with Barrett's esophagus. World J Gastroenterol 2017;23:3174-83. [Crossref] [PubMed]
- Hvid-Jensen F, Pedersen L, Funch-Jensen P, et al. Proton pump inhibitor use may not prevent high-grade dysplasia and oesophageal adenocarcinoma in Barrett's oesophagus: a nationwide study of 9883 patients. Aliment Pharmacol Ther 2014;39:984-91. [Crossref] [PubMed]
- Kia L, Komanduri S. Care of the Post-ablation Patient: Surveillance, Acid Suppression, and Treatment of Recurrence. Gastrointest Endosc Clin N Am 2017;27:515-29. [Crossref] [PubMed]
- Skrobić O, Simic A, Radovanovic N, et al. Significance of Nissen fundoplication after endoscopic radiofrequency ablation of Barrett's esophagus. Surg Endosc 2016;30:3802-7. [Crossref] [PubMed]
- Rayner CJ, Gatenby P. Effect of antireflux surgery for Barrett's esophagus: long-term results. Minerva Chir 2016;71:180-91. [PubMed]
- Krishnamoorthi R, Singh S, Ragunathan K, et al. Risk of recurrence of Barrett's esophagus after successful endoscopic therapy. Gastrointest Endosc 2016;83:1090-1106.e3. [Crossref] [PubMed]
- Felsenreich DM, Kefurt R, Schermann M, et al. Reflux, Sleeve Dilation, and Barrett's Esophagus after Laparoscopic Sleeve Gastrectomy: Long-Term Follow-Up. Obes Surg 2017;27:3092-101. [Crossref] [PubMed]
- Schlottmann F, Patti MG, Shaheen NJ. Endoscopic Treatment of High-Grade Dysplasia and Early Esophageal Cancer. World J Surg 2017;41:1705-11. [Crossref] [PubMed]
- Wolfsen HC, Hemminger LL, DeVault KR. Recurrent Barrett's esophagus and adenocarcinoma after esophagectomy. BMC Gastroenterol 2004;4:18. [Crossref] [PubMed]
- Lindblad M, Bright T, Schloithe A, et al. Toward More Efficient Surveillance of Barrett's Esophagus: Identification and Exclusion of Patients at Low Risk of Cancer. World J Surg 2017;41:1023-34. [Crossref] [PubMed]
- Realdon S, Antonello A, Arcidiacono D, et al. Adherence to WCRF/AICR lifestyle recommendations for cancer prevention and the risk of Barrett's esophagus onset and evolution to esophageal adenocarcinoma: results from a pilot study in a high-risk population. Eur J Nutr 2016;55:1563-71. [Crossref] [PubMed]
- Westerveld D, Khullar V, Mramba L, et al. Adherence to quality indicators and surveillance guidelines in the management of Barrett's esophagus: a retrospective analysis. Endosc Int Open 2018;6:E300-7. [Crossref] [PubMed]
- Cassani L, Slaughter JC, Yachimski P. Adherence to therapy for Barrett's esophagus-associated neoplasia. United European Gastroenterol J 2016;4:42-8. [Crossref] [PubMed]
- Britton J, Keld R, Prasad N, et al. Effect of diagnosis, surveillance, and treatment of Barrett's oesophagus on health-related quality of life. Lancet Gastroenterol Hepatol 2018;3:57-65. [Crossref] [PubMed]
- Chang CY, Lee LJ, Wang JD, et al. Health-related quality of life in patients with Barrett's esophagus. Health Qual Life Outcomes 2016;14:158. [Crossref] [PubMed]
- Kinsinger S. Elevated cancer risk perceptions among patients with Barrett's esophagus: do psychological factors play a role? Dis Esophagus 2018;31. [Crossref] [PubMed]
Cite this article as: Triadafilopoulos G, Friedland S. Precision care for Barrett’s esophagus. Transl Gastroenterol Hepatol 2018;3:67.


