Role of associating liver partition and portal vein ligation in staged hepatectomy (ALPPS)—strategy for colorectal liver metastases
Introduction
Colorectal carcinoma (CRC) is the third leading cause of cancer-related death in the United States (1). Almost 30% to 50% of patients with CRC develop liver metastases at sometime during the course of their disease (2,3). The extent of liver disease is a key determinant of survival in patients with isolated colorectal liver metastasis (CRLM). Although surgical resection remains the cornerstone of potentially curative therapy for patients with CRLM, few patients (20–30%) are amenable to surgical resection at the time of presentation either due to tumor burden or non-resectable extrahepatic metastases (4). Maintaining adequate future liver remnant (FLR) volume to avoid post-hepatectomy liver failure (PHLF), while achieving R0 resection is the main technical challenge especially in patients with bilobar CRLM.
The potential of hepatocytes to proliferate with hemodynamic changes, particularly an increase in portal venous flow, has led to development of a number of procedures aimed at increasing the volume and function of the FLR (5,6). Techniques such as portal vein embolization/ligation (PVE/PVL) followed by two-stage hepatectomy (TSH) have been established as standard procedures in management of patients with extensive metastatic liver disease. In 2007, Schlitt innovated a novel two-stage liver resection technique with the capability to rapidly enhance FLR volume before completion of surgical resection (7). Subsequently, Schnitzbauer et al. published the first clinical series on this novel technique in 2012 (8). Ultimately, the technique was named “associating liver partition and portal vein ligation for staged hepatectomy” (ALPPS) by de Santibañes et al. (7). We herein, reviewed the current role of ALPPS in the management of patients with CRLM.
Original surgical technique and subsequent modifications
ALPPS is a two-step procedure (Figure 1). In right lobe dominant disease, in the first step, the liver is completely mobilized and the right portal vein branch is ligated. Subsequently, the liver parenchyma is transected along the falciform ligament. Wedge resection of any metastatic lesions in the FLR (segments I/II/III) is performed in the first step as well. The right liver lobe and segment IV are then typically placed in a plastic bag to avoid post-operative adhesions during the waiting period until the next operation, and the abdomen is closed. After an interval of 7–14 days, the growth of the FLR is assessed by CT volumetry. If the volume gain seems sufficient, the patient undergoes the second step, usually involving a right tri-sectionectomy. The biliary tract reconstruction, if needed, is performed with a Roux-en-Y hepaticojejunostomy (8). In patients with synchronous CRLM, the primary tumor is often resected during first-stage of ALPPS (9).
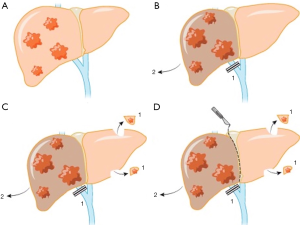
Various modifications of ALPPS have been reported in the literature, as more hepatobiliary centers started to adopt the procedure. In associating liver tourniquet and portal vein ligation for staged hepatectomy (ALTPS) introduced by Robles et al., instead of in situ splitting of the liver, a tourniquet was fixed along the future resection line to minimize the blood flow between the lobes (10). ALTPS was proposed to simplify the first step with reduced rate of adhesions and complications. The “anterior approach” was another modification that was suggested to minimize the peri-hepatic inflammation following the first step and hypothetically reduce the chance of hematologic spread of the malignancy (11). In the “anterior approach” complete liver mobilization was avoided and the hepatoduodenal ligament remained intact. Complete liver mobilization as well as dissection of all collateral flows ensures enough operative field exposure with enhanced rate of FLR hypertrophy, however, recent studies recommend minimal dissection of hepatoduodenal ligament to reduce the risk of both biliary leakage and segment 4 ischemia (12,13).
Several other technical modifications of ALPPS, according to the liver segment(s) that will comprise the FLR, have also been reported (14,15). In mono-segmental ALPPS, only one segment comprises the FLR (16,17). “Rescue ALPPS” refers to the ALPPS procedure performed in patients who did not achieve sufficient FLR following PVE to undergo the second step of TSH (18-21). ALPPS is considered as a last resort to induce liver hypertrophy in these patients. More recently, “laparoscopic first-stage ALPPS”, “totally laparoscopic ALPPS” and “robotic ALPPS” are minimally invasive approaches that have been reported to reduce the rate of postoperative adhesions and overall complications of the procedure (22-27).
Role of ALPPS in CRLM
Although ALPPS was initially reported in a patient with perihilar cholangiocarcinoma, shortly thereafter, CRLM patients constituted a considerable proportion of the candidates for this procedure (7,8,28) (Table 1).

Full table
In the first report of the international ALPPS registry of 202 patients, individuals with CRLM (n=141) had better prognosis compared to patients (n=61) with non-CRLM underlying diseases (29). The lower regenerative capacity of liver parenchyma and hence reduced regenerative capacity of the FLR in patients with primary liver malignancies, especially in the presence of fibrosis, might be the reason for the better outcomes of ALPPS among CRLM patients, who more often have normal liver function (29,32).
Recent advances in preoperative liver hypertrophy induction techniques and effective chemotherapy regimens, along with the advent of parenchymal-preserving liver surgery have expanded the limits of resectability in CRLM patients who were initially deemed to not be amenable to surgical resection because of insufficient FLR (3). Indeed, the ALPPS procedure has pushed the envelope even further and is a recommended choice of treatment in CRLM patients by the first ALPPS international expert meeting panel (33).
FLR hypertrophy
ALPPS may indeed be superior over PVE/PVL in inducing FLR hypertrophy in a shorter time interval. The liver hypertrophy in CRLM patients undergoing ALPPS has been reported to be as high as 110.3% in 7–14 days interval vs. 20–46% in 2–8 weeks following PVE (34,35). In a meta-analysis by Moris et al., while preoperative FLR, the extent of FLR increase, and postoperative FLR were all similar between the two groups, the kinetic growth rate was faster with ALPPS vs. PVE (mean difference: 19.07 mL/day; 95% CI: 8.12–30.02, P=0.0006) (36). The technical differences between the two procedures and the associated underlying physiologic effects on hepatocytes have been proposed as potential causes of the differences in hypertrophy. Partitioning the liver in the first stage prevents collateral vessels flow between the two sides of the liver and maintains the shear stress of the portal flow on hepatocytes at its maximal level, a known physiologic factor contributing to liver regeneration (34,37). Moreover, traumatizing the liver parenchyma during the first step of ALPPS might augment the inflammatory mediators triggering hepatocytes’ regeneration process (37). Of note, some investigators have suggested that liver hypertrophy at 1 week does not necessarily guarantee functional capacity, and may reflect edema rather than true hypertrophy and new hepatocyte proliferation. As such, assessing the function of the FLR, with methods beyond routine biochemical liver profile, rather than just the size should be strongly considered (34,38).
Selective internal radiation therapy (SIRT), also known as radioembolization (RE) is a technique developed to deliver Yttrium-90 (Y-90)-labeled microspheres to hepatic tumors via the hepatic artery. In recent years, RE has evolved as an effective treatment in selected patients with liver malignancies not amenable to surgical resection. In addition to tumoricidal effects, the RE has been shown to induce contralateral liver lobe hypertrophy as well. The reported contralateral liver hypertrophy following the Y-90 RE ranges between 26–47% in a time interval of 44 days to 9 months (39,40). The slower rate of hypertrophy is the potential important limitation of considering Y-90 RE as a pre-surgical strategy. There is growing evidence in the literature that this approach could be considered for patients with large unresectable tumors, who may benefit from dual downsizing/control of the tumor as well as FLR hypertrophy (40,41).
Feasibility of completion hepatectomy
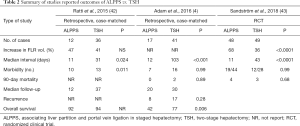
Another potential advantage of ALPPS over conventional TSH is higher feasibility for completion of both stages of the hepatectomy (Table 2).
Full table
Almost one-third of patients undergoing PVE/PVL followed by TSH for CRLM failed to complete the second stage resection with a feasibility of 65–75% (44). In contrast, a meta-analysis of patients with various underlying liver diseases who underwent ALPPS reported a completion hepatectomy feasibility of 97% (45). This proportion has increased to as high as 100% in studies including only CRLM patients (4,13,31). For example, in a study by Björnsson et al. the feasibility was 100% despite the fact that 43% of patients (n=10) underwent “rescue ALPPS” (31). Similarly, in a recent meta-analysis of 9 studies comparing ALPPS with TSH, the proportion of curative-intent resection, as well as the likelihood of proceeding to the second stage was higher in the ALPPS group compared with TSH [90.9% vs. 74.6%, respectively; risk ratio (RR) 1.21; 95% CI: 1.01–1.45, P=0.03] (36).
Tumor progression during time interval between two stages as well as insufficient FLR hypertrophy have been reported as two leading causes of patient drop out prior to completion hepatectomy in conventional TSH (44). While the shorter time prior to the second hepatectomy may increase overall feasibility, the shorter time interval between two stages in ALPPS might also hamper evaluation of the tumor biology and detection of micro-metastases that were not evident on initial imaging studies (46). This hypothesis has been supported by the relatively higher incidence of tumor recurrence and lower rate of disease-free survival (DFS) among patients undergoing ALPPS compared with conventional TSH (4,30).
Morbidity and mortality
PHLF, biliary leak and sepsis are serious complications of any liver resection. Current data comparing postoperative complications among CRLM patients undergoing ALPPS vs. conventional TSH has been contradictory. Ratti et al. compared outcomes of 12 ALPPS patients with 36 conventional TSH patients and reported that complications in the ALPPS group were higher than the TSH group (83.3% vs. 38.2%, respectively; P=0.011). There was one death reported in each group. No PHLF was observed in the ALPPS group, while two patients in the TSH group experienced liver failure. One-year overall survival (OS) and DFS were 92% and 67% in the ALPPS group vs. 94% and 80% in TSH group, respectively. In a different study by Adam et al. of 17 ALPPS and 41 conventional TSH patients, the incidence of major complications was no different between the two groups (41% vs. 39%, respectively; P=0.999). The 2-year OS of 42% in the ALPPS group was, however, lower than in the TSH group (77%, P=0.006); in contrast, DFS was comparable (4,42). Olthof et al. reported that the 2-year OS of patients with CRLM who underwent ALPPS depended on the liver tumor burden and varied between 49% and 72% (47). Interestingly, the OS of a group of patients with extensive liver disease who underwent ALPPS was comparable with a matched group of patients with unresectable CRLM who were treated with palliative chemotherapy (24 vs. 17.6 months, P=0.088). In the Björnsson et al. study of 23 patients with CRLM, the 2-year OS was 59% and severe complications occurred in 13.6% of patients (31).
In the first international registry study, the 90-day postoperative mortality among all patients who underwent ALPPS with different underlying liver diseases was 9% (29). For patients with CRLM, the 2-year OS and DFS were 62% and 41%, respectively. Of note, CRLM patients younger than 60 years had a better survival than patients with other pathologies. In as a study of fourteen patients with CRLM who underwent ALPPS, Hernandez-Alejandro et al. reported that OS was 100%, but median follow-up was only 9 months (13). Severe complications developed in only two patients. Moreover, only two patients experienced recurrence within 9 months. A separate analysis of the international ALPPS registry included 228 patients with CRLM, which comprised 72% of the study population. In this report, the 90-day mortality among CRLM patients who underwent ALPPS was 5% (48). The leading cause of mortality in patients with various underlying liver pathologies was “liver-related”. In turn, the authors suggested that the model of end-stage liver disease (MELD) score and the international study group for liver surgery (ISGLS) criteria could be utilized to discriminate between high and low risk patients. Developing liver failure following the first stage hepatectomy and MELD score of 10 or more prior to the second stage were independent predictors of 90-day mortality. The critical point to be considered in interpretation of any data comparing postoperative outcomes of these two procedures is that conventional TSH has typically been performed in younger CRLM patients, which are predictors of favorable outcome in ALPPS patients. However, current ALPPS data includes a substantial number of high-risk older patients with primary liver malignancies. The reported mortality rate of ALPPS for CRLM patients in general and for CRLM patients younger than 60 years of age were 8% and 5%, respectively, which are comparable to the reported mortality of TSH (49,50).
Stratification of patients for development of liver failure following the first stage hepatectomy, achieving expertise in the learning curve, refinements in patient selection, modifications applied to the original ALPPS procedure to decrease liver traumatization, interval chemotherapy, shorter time off chemotherapy, and preservation of segment 4 are some of the suggested approaches to enhance ALPPS outcomes in CRLM patients. Clinical studies with larger number of cases are required to confirm and better evaluate these possible improvements to the ALPPS approach.
Deciding whether ALPPS may be best option for patients with CRLM
Recently, Sandström et al. published the first multicenter randomized controlled trial comparing ALPPS and conventional TSH in patients with advanced CRLM (43). Patients with CRLM and FLR <30% were randomly assigned to ALPPS (n=48 patients) and TSH (n=49 patients). The primary outcome of successful resection of all liver disease was higher in ALPPS vs. TSH group (92% vs. 57%, respectively; P<0.0001). The incidence of major complications was the same in both groups (43%, P=0.99). Five patients (11%) in the ALPPS group underwent reoperation due to intestinal obstruction, wound rupture, and bile leak, while only one patient (3%) required reoperation in the TSH group due to intestinal obstruction (P=0.25). Notably, 13 patients (27%) in the TSH group dropped out before proceeding to the second stage hepatectomy. Twelve patients out of these 13 patients underwent rescue ALPPS, while the other one patient had tumor progression that prevented further surgery. After including these 12 “rescue” ALPPS cases in the TSH group, the resection rate in TSH group was 82% vs. 92% in the ALPPS group, although the difference was not statistically significant (P<0.1). Ninety-day mortality in the ALPPS (9.1%) and TSH (10.7%) groups was comparable (P=0.64). Despite the results on this one small-randomized study, ALPPS should generally not be considered as first line treatment for patients anticipated to achieve an adequate FLR with conventional PVE/PVL techniques. Rather, ALPPS should be reserved as an adjunct procedure for that subset of patients deemed to be not amenable to surgical resection with conventional procedures.
Conclusions
ALPPS has been reported to have a high feasibility and markedly induce FLR hypertrophy, which can broaden the eligibility of patients who have extensive tumor disease for surgical resection. In selected patients with extensive CRLM, ALPPS has acceptable morbidity and mortality compared with TSH. While conventional approaches such as PVE/PVL and TSH will generally be applicable in most patients with extensive CRLM, ALPPS may have a role in a subset of patients to increase the number of patients eligible for surgical resection.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018;68:7-30. [Crossref] [PubMed]
- de Jong MC, Pulitano C, Ribero D, et al. Rates and patterns of recurrence following curative intent surgery for colorectal liver metastasis: an international multi-institutional analysis of 1669 patients. Ann Surg 2009;250:440-8. [PubMed]
- Abdalla EK, Adam R, Bilchik AJ, et al. Improving resectability of hepatic colorectal metastases: expert consensus statement. Ann Surg Oncol 2006;13:1271-80. [Crossref] [PubMed]
- Adam R, Imai K, Castro Benitez C, et al. Outcome after associating liver partition and portal vein ligation for staged hepatectomy and conventional two-stage hepatectomy for colorectal liver metastases. Br J Surg 2016;103:1521-9. [Crossref] [PubMed]
- Riehle KJ, Dan YY, Campbell JS, et al. New concepts in liver regeneration. J Gastroenterol Hepatol 2011;26:203-12. [Crossref] [PubMed]
- Yokoyama Y, Nagino M, Nimura Y. Mechanisms of hepatic regeneration following portal vein embolization and partial hepatectomy: a review. World J Surg 2007;31:367-74. [Crossref] [PubMed]
- de Santibañes E, Clavien PA. Playing Play-Doh to prevent postoperative liver failure: the "ALPPS" approach. Ann Surg 2012;255:415-7. [Crossref] [PubMed]
- Schnitzbauer AA, Lang SA, Goessmann H, et al. Right portal vein ligation combined with in situ splitting induces rapid left lateral liver lobe hypertrophy enabling 2-staged extended right hepatic resection in small-for-size settings. Ann Surg 2012;255:405-14. [Crossref] [PubMed]
- Choi YI, Moon HH, Shin DH. Two cases of ALPPS procedure: simultaneous ALPPS and colorectal resection and ALPPS procedure for hepatic malignancy larger than 15 centimeter. Ann Hepatobiliary Pancreat Surg 2017;21:151-6. [Crossref] [PubMed]
- Robles R, Parrilla P, López‐Conesa A, et al. Tourniquet modification of the associating liver partition and portal ligation for staged hepatectomy procedure. Br J Surg 2014;101:1129-34. [Crossref] [PubMed]
- Chan AC, Pang R, Poon RT. Simplifying the ALPPS procedure by the anterior approach. Ann Surg 2014;260. [Crossref] [PubMed]
- Donati M, Stavrou GA, Oldhafer KJ. Current position of ALPPS in the surgical landscape of CRLM treatment proposals. World Journal of Gastroenterology: WJG 2013;19:6548. [Crossref] [PubMed]
- Hernandez-Alejandro R, Bertens KA, Pineda-Solis K, et al. Can we improve the morbidity and mortality associated with the associating liver partition with portal vein ligation for staged hepatectomy (ALPPS) procedure in the management of colorectal liver metastases? Surgery 2015;157:194-201. [Crossref] [PubMed]
- Gauzolino R, Castagnet M, Blanleuil ML, et al. The ALPPS technique for bilateral colorectal metastases: three “variations on a theme”. Updates Surg 2013;65:141-8. [Crossref] [PubMed]
- de Santibañes M, Alvarez FA, Santos FR, et al. The associating liver partition and portal vein ligation for staged hepatectomy approach using only segments I and IV as future liver remnant. J Am Coll Surg 2014;219:e5-9. [Crossref] [PubMed]
- Schadde E, Malagó M, Hernandez-Alejandro R, et al. Monosegment ALPPS hepatectomy: extending resectability by rapid hypertrophy. Surgery 2015;157:676-89. [Crossref] [PubMed]
- Steinbrück K, D'Oliveira M, Cano R, et al. Monosegmental ALPPS after Bilateral Hepatectomy. Ann Hepatol 2017;16:814-7. [PubMed]
- Maulat C, Suc B, Muscari F. Rescue ALPPS after portal embolization for colorectal metastases (with video). J Visc Surg 2018;155:77-8. [Crossref] [PubMed]
- Björnsson B, Gasslander T, Sandström P. In situ split of the liver when portal venous embolization fails to induce hypertrophy: a report of two cases. Case Rep Surg 2013;2013.
- Vyas SJ, Davies N, Grant L, et al. Failure of portal venous embolization. ALPPS as salvage enabling successful resection of bilobar liver metastases. J Gastrointest Cancer 2014;45:233-6. [Crossref] [PubMed]
- Tschuor C, Croome K, Sergeant G, et al. Salvage parenchymal liver transection for patients with insufficient volume increase after portal vein occlusion–an extension of the ALPPS approach. Eur J Surg Oncol 2013;39:1230-5. [Crossref] [PubMed]
- Machado MA, Makdissi FF, Surjan RC. Totally laparoscopic ALPPS is feasible and may be worthwhile. Ann Surg 2012;256. [Crossref] [PubMed]
- Machado MA, Makdissi FF, Surjan RC, et al. Transition from open to laparoscopic ALPPS for patients with very small FLR: the initial experience. HPB 2017;19:59-66. [Crossref] [PubMed]
- Conrad C, Shivathirthan N, Camerlo A, et al. Laparoscopic portal vein ligation with in situ liver split for failed portal vein embolization. Ann Surg 2012;256:e14-5. [Crossref] [PubMed]
- Vicente E, Quijano Y, Ielpo B, et al. First ALPPS procedure using a total robotic approach. Surg Oncol 2016;25:457. [Crossref] [PubMed]
- Gringeri E, Boetto R, D'Amico FE, et al. Laparoscopic microwave ablation and portal vein ligation for staged hepatectomy (LAPS): a minimally invasive first-step approach. Ann Surg 2015;261:e42-3. [Crossref] [PubMed]
- Machado M, Surjan R, Basseres T, et al. Total laparoscopic reversal ALPPS. Ann Surg Oncol 2017;24:1048-9. [Crossref] [PubMed]
- Nadalin S, Capobianco I, Li J, et al. Indications and limits for associating liver partition and portal vein ligation for staged hepatectomy (ALPPS). Lessons learned from 15 cases at a single centre. Z Gastroenterol 2014;52:35-42. [Crossref] [PubMed]
- Schadde E, Ardiles V, Robles-Campos R, et al. Early survival and safety of ALPPS: first report of the International ALPPS Registry. Ann Surg 2014;260:829-36; discussion 836-8. [Crossref]
- Oldhafer KJ, Donati M, Jenner RM, et al. ALPPS for patients with colorectal liver metastases: effective liver hypertrophy, but early tumor recurrence. World J Surg 2014;38:1504-9. [Crossref] [PubMed]
- Björnsson B, Sparrelid E, Røsok B, et al. Associating liver partition and portal vein ligation for staged hepatectomy in patients with colorectal liver metastases–intermediate oncological results. Eur J Surg Oncol 2016;42:531-7. [Crossref] [PubMed]
- Cai YL, Song PP, Tang W, et al. An updated systematic review of the evolution of ALPPS and evaluation of its advantages and disadvantages in accordance with current evidence. Medicine (Baltimore) 2016;95. [Crossref] [PubMed]
- Oldhafer KJ, Stavrou GA, van Gulik TM. ALPPS—where do we stand, where do we go?: eight recommendations from the first international expert meeting. Ann Surg 2016;263:839-41. [Crossref] [PubMed]
- Tanaka K, Endo I. ALPPS: short-term outcome and functional changes in the future liver remnant. Ann Surg 2015;262:e88-9. [Crossref] [PubMed]
- Ielpo B, Caruso R, Ferri V, et al. ALPPS procedure: our experience and state of the art. Hepato-gastroenterology 2013;60:2069-75. [PubMed]
- Moris D, Ronnekleiv-Kelly S, Kostakis ID, et al. Operative Results and Oncologic Outcomes of Associating Liver Partition and Portal Vein Ligation for Staged Hepatectomy (ALPPS) Versus Two-Stage Hepatectomy (TSH) in Patients with Unresectable Colorectal Liver Metastases: A Systematic Review and Meta-Analysis. World J Surg 2018;42:806-15. [Crossref] [PubMed]
- Gall TM, Sodergren MH, Frampton AE, et al. Radio-frequency-assisted liver partition with portal vein ligation (RALPP) for liver regeneration. Ann Surg 2015;261:e45-6. [Crossref] [PubMed]
- Oldhafer F, Ringe KI, Timrott K, et al. Monitoring of liver function in a 73-year old patient undergoing ‘Associating Liver Partition and Portal vein ligation for Staged hepatectomy’: case report applying the novel liver maximum function capacity test. Patient Saf Surg 2016;10:16. [Crossref] [PubMed]
- Teo JY, Allen JC Jr, Ng DC, et al. A systematic review of contralateral liver lobe hypertrophy after unilobar selective internal radiation therapy with Y90. HPB (Oxford) 2016;18:7-12. [Crossref] [PubMed]
- Orcutt ST, Abuodeh Y, Naghavi A, et al. Kinetic analysis of contralateral liver hypertrophy after radioembolization of primary and metastatic liver tumors. Surgery 2018;163:1020-7. [Crossref] [PubMed]
- Bouazza F, Poncelet A, Garcia CA, et al. Radioembolisation and portal vein embolization before resection of large hepatocellular carcinoma. World J Gastroenterol 2015;21:9666-70. [Crossref] [PubMed]
- Ratti F, Schadde E, Masetti M, et al. Strategies to increase the resectability of patients with colorectal liver metastases: a multi-center case-match analysis of ALPPS and conventional two-stage hepatectomy. Ann Surg Oncol 2015;22:1933-42. [Crossref] [PubMed]
- Sandström P, Røsok BI, Sparrelid E, et al. ALPPS improves resectability compared with conventional two-stage hepatectomy in patients with advanced colorectal liver metastasis: results from a Scandinavian multicenter randomized controlled trial (LIGRO trial). Ann Surg 2018;267:833. [PubMed]
- Lam VW, Laurence JM, Johnston E, et al. A systematic review of two-stage hepatectomy in patients with initially unresectable colorectal liver metastases. HPB 2013;15:483-91. [Crossref] [PubMed]
- Schadde E, Schnitzbauer AA, Tschuor C, et al. Systematic review and meta-analysis of feasibility, safety, and efficacy of a novel procedure: associating liver partition and portal vein ligation for staged hepatectomy. Ann Surg Oncol 2015;22:3109-20. [Crossref] [PubMed]
- Lang H. ALPPS for Colorectal Liver Metastases. J Gastrointest Surg 2017;21:190-2. [Crossref] [PubMed]
- Olthof PB, Huiskens J, Wicherts DA, et al. Survival after associating liver partition and portal vein ligation for staged hepatectomy (ALPPS) for advanced colorectal liver metastases: a case-matched comparison with palliative systemic therapy. Surgery 2017;161:909-19. [Crossref] [PubMed]
- Schadde E, Raptis DA, Schnitzbauer AA, et al. Prediction of mortality after ALPPS stage-1: an analysis of 320 patients from the International ALPPS Registry. Ann Surg 2015;262:780-5; discussion 785-6. [Crossref]
- Brouquet A, Abdalla EK, Kopetz S, et al. High survival rate after two-stage resection of advanced colorectal liver metastases: response-based selection and complete resection define outcome. J Clin Oncol 2011;29:1083-90. [Crossref] [PubMed]
- Wicherts DA, Miller R, de Haas RJ, et al. Long-term results of two-stage hepatectomy for irresectable colorectal cancer liver metastases. Ann Surg 2008;248:994-1005. [Crossref] [PubMed]
Cite this article as: Abbasi A, Rahnemai-Azar AA, Merath K, Weber SM, Abbott DE, Dillhoff M, Cloyd J, Pawlik TM. Role of associating liver partition and portal vein ligation in staged hepatectomy (ALPPS)—strategy for colorectal liver metastases. Transl Gastroenterol Hepatol 2018;3:66.


