Transplantation for hepatocellular cancer: pushing to the limits?
Introduction
Milan criteria (MC) represents the cornerstone in the selection of patients with hepatocellular cancer (HCC) waiting for liver transplantation (LT) (1). After their introduction in 1996, several new scoring systems have been proposed with the intent to push beyond the patient selection limits (2). In fact, although the MC consent to obtain excellent post-LT survival rates, they are very restrictive, thus impeding to transplant a significant number of subjects having border-line tumoral conditions.
However, when we need to identify the best selection model to use for selecting HCC patients, typically we think at a score contemporaneously able to improve the number of transplantable cases and to maintain a low rate of HCC-specific death or recurrence (3). Such a scheme, typically used for constructing the vast majority of the proposed West and East scoring systems, is substantially based on the idea of “utility” (4-8).
However, some other scores have been proposed specifically investigating the risk of death or tumour progression during the waiting list (drop-out, DO) (9-11). In this case, the selection process is connected with the idea of “priority”: patients at higher risk for DO should be selected, prioritising them or, conversely, deciding to de-list them due to the high risk of post-LT futile transplant.
Lastly, models based on the concept of benefit have been recently created (12-15). The transplant benefit represents a compromise between utility and priority, trying to define the group of patients better really obtaining a beneficial effect concerning survival after LT (16).
The present review aims to examine these three different types of scoring systems, trying to underline their pro and cons in the allocation process of HCC patients.
Utility models: from the dictature of morphology to the realm of biology
In 1996, Mazzaferro et al. proposed the so-called MC, constructing a model based on the combination of number and dimension of the tumoral burden (1). Afterwards, several new morphology-based scores were proposed, all of them trying to slightly enlarge the number of potentially transplantable patients without contemporaneously decreasing the remarkable survivals obtained using the MC (Table 1) (2,4,6-8). In 2009, Mazzaferro et al. proposed a new model derived from the sum of number and dimensions of a tumour: the up-to-seven criteria (5), However, in the same period, growing evidence started raising on the opportunity to combine morphological and biological elements, with the main intent to improve the patient selection. In 2010, an Editorial by Marsh et al. interestingly stated that: “…in the field of surgical oncology, tumour biology is king, patient selection is queen, and technical manoeuvres are the prince and princess who try, but usually fail, to usurp the throne.” Among the different biology-related variables, alpha-fetoprotein (AFP) was the most commonly analysed feature (17-23,28,35). Its absolute value available immediately before the transplant was correlated with a higher risk for post-LT recurrence (35). Its slope was similarly connected with an improved ability in selecting the risk for recurrence or intention-to-treat (ITT) death, even if they were within the MC (17,28). Several cut-offs were tested, with the most commonly proposed ones being 400 or 1,000 ng/mL (18-20). Studies exploring the combination of AFP with other radiological and biological aspects were proposed (17-23). As an example, three scoring systems investigating the integration between radiological aspects and AFP were recently proposed and validated (21-23). Another biological marker analysed in combination with AFP or alone was the des-gamma-carboxy prothrombin (DCP) (24,25,36). A recent study from Korea has shown that the combination of DCP and AFP consented to improve the prognostic ability in living-donor LT subjects (25). A meta-analysis confirmed the prognostic role of DCP, with a 5-fold increased risk of recurrence after LT (36).
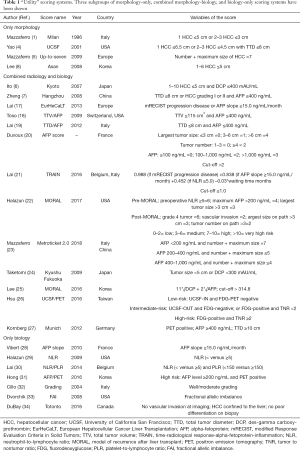
Full table
The role of inflammatory markers has also been investigated in the specific field of LT. As an example, the neutrophil-to-lymphocyte ratio has been evaluated as a single prognostic tool or after its integration with other variables, showing great ability in the prediction of ITT survival and post-LT recurrence (21,22,29,30). Similarly, the platelet-to-lymphocyte ratio has shown a good prognostic ability in a recent meta-analysis, reporting a 3-fold increased risk for recurrence in patients having high values of this ratio (37).
The radiological response to loco-regional treatments has been similarly investigated. Progression disease has been proposed as an important selection tool in recent studies coming from Europe, Asia and the US (17,21,38,39). Studies from Asia and Europe also investigated the uptake of the F-18 fluorodeoxyglucose (FDG) after positron emission tomography (PET), combining this element with the radiological aspects of the tumour (26,27,31).
Some studies proposed to perform a pre-LT biopsy with the intent to select the patients according to the histological features of the tumor (32-34). Among them, a study from Padua underlined the importance of the tumor grading, showing that transplanting patients with well-to-moderated grading even exceeding the MC was connected with acceptable survivals (32).
A study from the US identified the fractional allelic imbalance rate index as an important discriminating tool for selecting patients at high risk for HCC recurrence after transplant. The fractional allelic imbalance was found to be the strongest predictor of recurrence, followed by vascular invasion, tumor number, and hepatic lobar involvement (33).
The recently proposed Toronto criteria were based on three aspects: (I) no vascular invasion detected at imaging; (II) tumor exclusively located into the liver; and (III) no poor grading on biopsy. Comparing the results of 189 MC-IN vs. 105 Toronto criteria patients, no differences were observed in terms of 5-year overall (72% vs. 70%) and disease-free survivals (70% vs. 66%) (34).
Lastly, we should like to underline that several of the studies reported in this session mainly focused on the combination of different features (morphological + biological or biological + biological), thus proposing the idea that the different risk factors for recurrence or death may cause a cumulative effect (6,7,9,15,17-27).
Priority models: finding a model for equalizing the MELD score
The Model for End-stage Liver Disease (MELD) represents the best tool for selecting no-HCC patients waiting for LT. However, when we try to find a similar score able to select and prioritise patients having a tumour, we have the significant problem of trying to “equalise” these two different groups of patients. In fact, the main risk in this setting is to violate the rule of “equity”: in other terms, we favour a specific subclass, harming the other one regarding increased risk of DO.
In the US, the policy of adding MELD exception points for HCC individuals represents the typical strategy. However, several modifications have been done during the years, with the intent to reduce possible unbalances (40,41) (Table 2).
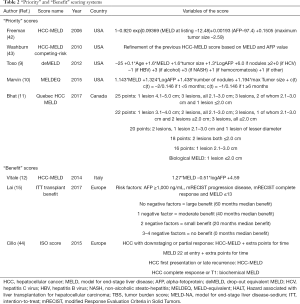
Full table
A first attempt to create a score able to consent an equitable liver allocation process was proposed in 2006: the HCC-MELD score was developed, showing that the factors associated with the risk of removal for HCC are different from non-tumour candidates (42). A statistical refinement of the score was performed some years later, performing a competing-risk analysis for the identification of the independent risk factors for de-listing: MELD and AFP value confirmed their important role in this setting (43).
In 2012, a large study from the US proposed the DO equivalent MELD (deMELD). This model was able to assess the risk of DO in patients with or without HCC, trying to find a better comparison of the opportunities of these patients of being transplanted. Unfortunately, the main limit of the present score is its complexity based on several tumour- and patient-related covariates (9).
Another study proposed the MELDEQ, in which the MELD points were combined with AFP, tumour number and tumour dimensions (10).
A study from Quebec, Canada, implemented the MELD model adding points according to several combinations of tumour diameters and nodules numbers: using this model, the transplant rates among HCC and no-HCC cases became equivalent, without compromising post-LT graft and patient survival (11).
A recent study coming from Cleveland (US), interestingly showed that the use of a continuous risk score able to longitudinally assess the risk for DO and ITT death was superior respect to dichotomous variables in determining a more granular estimation of the risk: also, in this case, MELD and tumour-related variables were integrated (45).
Although several aspects have been deeply investigated, some significant variables, like the different blood groups, have not been exhaustively indagated in terms of ability to modify the waiting time/demand for LT in the field of HCC (46). More studies are needed on this specific aspect.
Benefit models: a challenging balancing
The concept of transplant benefit expresses the survival gain obtained comparing LT with the best alternative therapies (i.e., a difference between life years obtained with and without LT). The transplant benefit used with a mid-term time horizon (post-transplant 5–10 years) has the inherent potential to reach the dignity of an independent LT selection principle (3).
In 2008, Volk et al. first investigated this concept, looking at the comparison between the survival benefit of transplanting an HCC patient exceeding the MC, versus the harm caused in no transplanting no-HCC patients on the waiting list. Interestingly, the harm outweighed the benefit of transplantation when the post-LT 5-year survival for HCC cases was <61%, with a threshold ranging 25–72% according to the different donation numerosity observed in the different areas (37).
Similar evidence was shown in a similar study performed in Italy, in which the high number of donors in the region determined a relatively low 5-year survival rate (only 30%) for consenting the transplant benefit to outweigh the harm (47).
Another study from Italy explored a large population of HCC patients with the intent to explore the transplant benefit in different subgroups according to the Barcelona Clinic Liver Cancer (BCLC) class. The median 5-year transplant benefit was 11.2 months for BCLC 0, 13.5 for BCLC A, 17.4 for BCLC B-C, and 28.5 for BCLC D, thus showing a growing benefit with the growing severity of liver dysfunction (48). Two recent “benefit-related” scores have been proposed (Table 2).
A study based on 2,697 enlisted and 1,702 transplanted subjects consented to develop the HCC-MELD score. The score consented to calculate a numerical score for HCC patients based on the combination of AFP and MELD, whereby consenting to obtain a transplant benefit equal to that of no-HCC patients with the same numerical value for MELD (12).
A multicentric European study performed on 2,103 HCC patients introduced the new concept of ITT transplant benefit, being able to estimate the benefit from the moment of waiting list inscription. Four risk factors for the development of a significant benefit were defined, namely AFP ≥1,000 ng/mL, mRECIST progression disease, mRECIST complete response e MELD ≤13. After using the combination of these four variables, it was possible to identify four groups of patients presenting large to no benefit (15). A recent score developed in Italy, the so-called “ISO Score” has been proposed with the intent to modify the liver allocation policy. A “mixed” approach has been proposed, in which the indicators for orienting organ allocation policies based on the principles of urgency, utility, and transplant benefit have been identified. MELD exceptions and HCC have been analyzed to construct a LT priority algorithm aimed at overpassing the inequity of a purely MELD-based system (44).
Considerations and conclusions
The decision to use a specific scoring system in selecting HCC patients deserving LT presents several challenging decisions to take. The first consideration to do is connected with the model to construct. A score must be easy to be used, but contemporaneously able to consent good discrimination. This exigence limits the possibility to use sophisticated models requiring several variables. All the reported scores typically use the same, limited, number of variables. MELD score is typically present in “priority” scores, being integrated with some tumour-related variables (i.e., AFP, tumour response, tumour morphology). For the “utility” scores, number and dimension of a tumour combined with some biological aspect (AFP or radiological response) represent the easier way of capturing the risks of recurrence or HCC-specific death. Typically, the cumulative effect of the different risk factors improves the prognostic ability of the model, as shown in different experiences (6,7,9,15,17-27).
For the “benefit” scores, as expected, a combination of “utility” and “priority” elements has been adequately reported (i.e., MELD, AFP, radiological response).
Another critical consideration is strictly connected with ethical issues. Which one of these selection scores should be preferred? As reported, the benefit represents the balancing between priority and utility. As a consequence, it should adequately represent the best way of constructing models based on the principle of equity, thus consenting to give the same opportunities to HCC and no-HCC patients. Unfortunately, the complexity of a waiting-list population is difficult to be easily captured by a user-friendly score. We retain a sort of compromise should be attempted, creating two different models. An easy-to-use “priority” score based on the combination of HCC-related features and MELD should be developed, aimed at selecting in an equal way tumour and non-tumour patients waiting in the list. Then, a similar “utility” score aimed at identifying patients at risk for HCC-specific death should be proposed, with the possible double intent of (I) excluding patients at high future risk of recurrence (futile transplant), or (II) following-up with greater attention identified high-risk cases after transplant.
Lastly, we should like to stress another important issue directly connected with the concepts of “inclusion” and “exclusion” from the LT. If, on one side, the exclusion of patients with a “too advanced” tumor looks to be intuitive, on the other the decision to treat with alternative approaches a patient with a tumor at very low risk for progression or recurrence represents a big deal. As an example, patients showing a complete biological/radiological response and a low MELD should remain for a long period in the waiting list presenting a very low risk for DO (15,49). Consequently, these patients may be de-listed and strictly followed-up for the risk of recurrence. However, all of the here reported issues require further studies with the intent to find the best way of equilibrating the severe imbalance existent in the allocation process of HCC patients.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med 1996;334:693-9. [Crossref] [PubMed]
- Toso C, Kneteman NM, James Shapiro AM, et al. The estimated number of patients with hepatocellular carcinoma selected for liver transplantation using expanded selection criteria. Transpl Int 2009;22:869-75. [Crossref] [PubMed]
- Vitale A, Lai Q. Selection of patients with hepatocellular cancer: a difficult balancing between equity, utility, and benefit. Transl Gastroenterol Hepatol 2017;2:75. [Crossref] [PubMed]
- Yao FY, Ferrell L, Bass NM, et al. Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology 2001;33:1394-403. [Crossref] [PubMed]
- Mazzaferro V, Llovet JM, Miceli R, et al. Metroticket Investigator Study Group. Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: a retrospective, exploratory analysis. Lancet Oncol 2009;10:35-43. [Crossref] [PubMed]
- Ito T, Takada Y, Ueda M, et al. Expansion of selection criteria for patients with hepatocellular carcinoma in living donor liver transplantation. Liver Transpl 2007;13:1637-44. [Crossref] [PubMed]
- Zheng SS, Xu X, Wu J, et al. Liver transplantation for hepatocellular carcinoma: Hangzhou experiences. Transplantation 2008;85:1726-32. [Crossref] [PubMed]
- Lee SG, Hwang S, Moon DB, et al. Expanded indication criteria of living donor liver transplantation for hepatocellular carcinoma at one large-volume center. Liver Transpl 2008;14:935-45. [Crossref] [PubMed]
- Toso C, Dupuis-Lozeron E, Majno P, et al. A model for dropout assessment of candidates with or without hepatocellular carcinoma on a common liver transplant waiting list. Hepatology 2012;56:149-56. [Crossref] [PubMed]
- Marvin MR, Ferguson N, Cannon RM, et al. MELDEQ: An alternative Model for End-Stage Liver Disease score for patients with hepatocellular carcinoma. Liver Transpl 2015;21:612-22. [Crossref] [PubMed]
- Bhat M, Ghali P, Dupont B, et al. Proposal of a novel MELD exception point system for hepatocellular carcinoma based on tumor characteristics and dynamics. J Hepatol 2017;66:374-81. [Crossref] [PubMed]
- Vitale A, Volk ML, De Feo TM, et al. Liver Transplantation North Italy Transplant program (NITp) working group. A method for establishing allocation equity among patients with and without hepatocellular carcinoma on a common liver transplant waiting list. J Hepatol 2014;60:290-7. [Crossref] [PubMed]
- Volk ML, Vijan S, Marrero JA. A novel model measuring the harm of transplanting hepatocellular carcinoma exceeding Milan criteria. Am J Transplant 2008;8:839-46. [Crossref] [PubMed]
- Vitale A, Huo TL, Cucchetti A, et al. Survival Benefit of Liver Transplantation Versus Resection for Hepatocellular Carcinoma: Impact of MELD Score. Ann Surg Oncol 2015;22:1901-7. [Crossref] [PubMed]
- Lai Q, Vitale A, Iesari S, et al. European Hepatocellular Cancer Liver Transplant Study Group. Intention-to-treat survival benefit of liver transplantation in patients with hepatocellular cancer. Hepatology 2017;66:1910-9. [Crossref] [PubMed]
- Marsh JW, Schmidt C. The Milan criteria: no room on the metro for the king? Liver Transpl 2010;16:252-5. [PubMed]
- Lai Q, Avolio AW, Graziadei I. wr al; European Hepatocellular Cancer Liver Transplant Study Group. Alpha-fetoprotein and modified response evaluation criteria in solid tumors progression after locoregional therapy as predictors of hepatocellular cancer recurrence and death after transplantation. Liver Transpl 2013;19:1108-18. [PubMed]
- Toso C, Meeberg G, Hernandez-Alejandro R, et al. Total tumor volume and alpha-fetoprotein for selection of transplant candidates with hepatocellular carcinoma: A prospective validation. Hepatology 2015;62:158-65. [Crossref] [PubMed]
- Lai Q, Avolio AW, Manzia TM, et al. Combination of biological and morphological parameters for the selection of patients with hepatocellular carcinoma waiting for liver transplantation. Clin Transplant 2012;26:E125-31. [Crossref] [PubMed]
- Duvoux C, Roudot-Thoraval F, Decaens T, et al. Liver Transplantation French Study Group. Liver transplantation for hepatocellular carcinoma: a model including α-fetoprotein improves the performance of Milan criteria. Gastroenterology 2012;143:986-94.e3. [Crossref] [PubMed]
- Lai Q, Nicolini D, Inostroza Nunez M, et al. A Novel Prognostic Index in Patients With Hepatocellular Cancer Waiting for Liver Transplantation: Time-Radiological-response-Alpha-fetoprotein-INflammation (TRAIN) Score. Ann Surg 2016;264:787-96. [Crossref] [PubMed]
- Halazun KJ, Najjar M, Abdelmessih RM, et al. Recurrence After Liver Transplantation for Hepatocellular Carcinoma: A New MORAL to the Story. Ann Surg 2017;265:557-64. [Crossref] [PubMed]
- Mazzaferro V, Sposito C, Zhou J, et al. Metroticket 2.0 Model for Analysis of Competing Risks of Death After Liver Transplantation for Hepatocellular Carcinoma. Gastroenterology 2018;154:128-39. [Crossref] [PubMed]
- Taketomi A, Sanefuji K, Soejima Y, et al. Impact of des-gamma-carboxy prothrombin and tumor size on the recurrence of hepatocellular carcinoma after living donor liver transplantation. Transplantation 2009;87:531-7. [Crossref] [PubMed]
- Lee JH, Cho Y, Kim HY, et al. Serum tumor markers provide refined prognostication in selecting liver transplantation candidate for hepatocellular carcinoma patients beyond the Milan Criteria. Ann Surg 2016;263:842-50. [Crossref] [PubMed]
- Hsu CC, Chen CL, Wang CC, et al. Combination of FDG-PET and UCSF Criteria for Predicting HCC Recurrence After Living Donor Liver Transplantation. Transplantation 2016;100:1925-32. [Crossref] [PubMed]
- Kornberg A, Küpper B, Tannapfel A, et al. Patients with non-[18 F]fludeoxyglucose-avid advanced hepatocellular carcinoma on clinical staging may achieve long-term recurrence-free survival after liver transplantation. Liver Transpl 2012;18:53-61. [Crossref] [PubMed]
- Vibert E, Azoulay D, Hoti E, et al. Progression of alphafetoprotein before liver transplantation for hepatocellular carcinoma in cirrhotic patients: a critical factor. Am J Transplant 2010;10:129-37. [Crossref] [PubMed]
- Halazun KJ, Hardy MA, Rana AA, et al. Negative impact of neutrophil-lymphocyte ratio on outcome after liver transplantation for hepatocellular carcinoma. Ann Surg 2009;250:141-51. [Crossref] [PubMed]
- Lai Q, Castro Santa E, Rico Juri JM, et al. Neutrophil and platelet-to-lymphocyte ratio as new predictors of dropout and recurrence after liver transplantation for hepatocellular cancer. Transpl Int 2014;27:32-41. [Crossref] [PubMed]
- Hong G, Suh KS, Suh SW, et al. Alpha-fetoprotein and (18)F-FDG positron emission tomography predict tumor recurrence better than Milan criteria in living donor liver transplantation. J Hepatol 2016;64:852-9. [Crossref] [PubMed]
- Cillo U, Vitale A, Bassanello M, et al. Liver transplantation for the treatment of moderately or well-differentiated hepatocellular carcinoma. Ann Surg 2004;239:150-9. [Crossref] [PubMed]
- Dvorchik I, Schwartz M, Fiel MI, et al. Fractional allelic imbalance could allow for the development of an equitable transplant selection policy for patients with hepatocellular carcinoma. Liver Transpl 2008;14:443-50. [Crossref] [PubMed]
- DuBay D, Sandroussi C, Sandhu L, et al. Liver transplantation for advanced hepatocellular carcinoma using poor tumor differentiation on biopsy as an exclusion criterion. Ann Surg 2011;253:166-72. [Crossref] [PubMed]
- Merani S, Majno P, Kneteman NM, et al. The impact of waiting list alpha-fetoprotein changes on the outcome of liver transplant for hepatocellular carcinoma. J Hepatol 2011;55:814-9. [Crossref] [PubMed]
- Lai Q, Iesari S, Levi Sandri GB, et al. Des-gamma-carboxy prothrombin in hepatocellular cancer patients waiting for liver transplant: a systematic review and meta-analysis. Int J Biol Markers 2017;32:e370-4. [Crossref] [PubMed]
- Lai Q, Melandro F, Larghi Laureiro Z, et al. Platelet-to-lymphocyte ratio in the setting of liver transplantation for hepatocellular cancer: A systematic review and meta-analysis. World J Gastroenterol 2018;24:1658-65. [Crossref] [PubMed]
- Kim DJ, Clark PJ, Heimbach J, et al. Recurrence of hepatocellular carcinoma: importance of mRECIST response to chemoembolization and tumor size. Am J Transplant 2014;14:1383-90. [Crossref] [PubMed]
- Agopian VG, Harlander-Locke MP, Ruiz RM, et al. Impact of Pretransplant Bridging Locoregional Therapy for Patients With Hepatocellular Carcinoma Within Milan Criteria Undergoing Liver Transplantation: Analysis of 3601 Patients From the US Multicenter HCC Transplant Consortium. Ann Surg 2017;266:525-35. [Crossref] [PubMed]
- Wedd JP, Nordstrom E, Nydam T, et al. Hepatocellular carcinoma in patients listed for liver transplantation: Current and future allocation policy and management strategies for the individual patient. Liver Transpl 2015;21:1543-52. [Crossref] [PubMed]
- Ioannou GN, Perkins JD, Carithers RL Jr. Liver transplantation for hepatocellular carcinoma: impact of the MELD allocation system and predictors of survival. Gastroenterology 2008;134:1342-51. [Crossref] [PubMed]
- Freeman RB, Edwards EB, Harper AM. Waiting list removal rates among patients with chronic and malignant liver diseases. Am J Transplant 2006;6:1416-21. [Crossref] [PubMed]
- Washburn K, Edwards E, Harper A, et al. Hepatocellular carcinoma patients are advantaged in the current liver transplant allocation system. Am J Transplant 2010;10:1643-8. [Crossref] [PubMed]
- Cillo U, Burra P, Mazzaferro V, et al. I-BELT (Italian Board of Experts in the Field of Liver Transplantation). A Multistep, Consensus-Based Approach to Organ Allocation in Liver Transplantation: Toward a "Blended Principle Model". Am J Transplant 2015;15:2552-61. [Crossref] [PubMed]
- Firl DJ, Kimura S, McVey J, et al. Reframing the approach to patients with hepatocellular carcinoma: Longitudinal assessment with HALT-HCC improves ablate and wait strategy. Hepatology 2018. [Epub ahead of print]. [Crossref] [PubMed]
- Barone M, Avolio AW, Di Leo A, et al. ABO blood group-related waiting list disparities in liver transplant candidates: effect of the MELD adoption. Transplantation 2008;85:844-9. [Crossref] [PubMed]
- Vitale A, Volk ML, Gambato M, et al. Estimation of the harm to the waiting list as a crucial factor in the selection of patients with hepatocellular carcinoma for liver transplantation. Transplant Proc 2010;42:1194-6. [Crossref] [PubMed]
- Vitale A, Morales RR, Zanus G, et al. Italian Liver Cancer group. Barcelona Clinic Liver Cancer staging and transplant survival benefit for patients with hepatocellular carcinoma: a multicentre, cohort study. Lancet Oncol 2011;12:654-62. [Crossref] [PubMed]
- Mehta N, Dodge JL, Goel A, et al. Identification of liver transplant candidates with hepatocellular carcinoma and a very low dropout risk: implications for the current organ allocation policy. Liver Transpl 2013;19:1343-53. [Crossref] [PubMed]
Cite this article as: Lai Q, Vitale A. Transplantation for hepatocellular cancer: pushing to the limits? Transl Gastroenterol Hepatol 2018;3:61.


