Resection for intrahepatic cholangiocellular cancer: new advances
Introduction
Cholangiocarcinoma is a heterogeneous group of malignant neoplasms that arise from the epithelial cells of the extrahepatic and intrahepatic bile ducts, excluding the gallbladder and ampulla of Vater. It is classically divided according to its anatomical location in intrahepatic (ICC)—arising from cells located proximal to the second-degree bile ducts—and extrahepatic cholangiocarcinoma (ECC), which can be further subdivided in perihilar (pCCA) and distal cholangiocarcinoma (dCCA) (1). This classification is important because ICC, pCCA and dCCA have divergent clinical presentation, epidemiological features and biological behavior. In fact, pCCA accounts for 60–70% of cases, dCCA for 20–30% and ICC for 5–10% (2).
Epidemiology
Among primary hepatic malignancies, ICC is the second most prevalent neoplasm after hepatocellular carcinoma (HCC), corresponding to 10% to 15% of cases (3). Nevertheless, it is still a rare tumor, representing 3% of gastrointestinal malignancies (4). On the other hand, its age-adjusted incidence has increased worldwide, from 0.32 per 100,000 to 0.85 per 100,000 between 1975 and 2000, which represents a rise of 165% (4). It is believed that this growing incidence is a result of a true increase in the disease, not being solely attributable to improvements in diagnostic methods (5).
Pathologies that cause chronic biliary inflammation and bile stasis are known predisposing factors for development of ICC, most commonly primary sclerosing cholangitis (PSC), intrahepatic lithiasis, congenital abnormalities of the biliary tract, parasite infections (Clonorchis sinensis and Opisthorchis viverrini) and toxic exposure (thorium dioxide) (6). Interestingly, chronic liver disease and especially hepatitis B and C virus have been recently recognized as risks factors for ICC (6,7). Metabolic abnormalities such as type II diabetes, obesity, thyrotoxicosis and chronic pancreatitis have also been linked to ICC (7). Nevertheless, most patients will present with ICC with no identifiable risk factor (5). Similar to other biliary tract malignancies, ICC incidence peaks between 55 and 75 years and is slightly more frequent in men (4,7).
Clinical and diagnostic features
Most patients remain asymptomatic until advance stage (8). Unlike ECC, which typically presents with jaundice and biliary obstruction, the common presentation of ICC is an incidental liver mass found on imaging studies performed for other reasons or during evaluation of abnormal liver enzymes tests (5). Patients may also complain of vague right upper abdominal pain and weight loss. Tumor markers, such as carbohydrate antigen (CA) 19-9 and carcinoembryonic antigen (CEA), may be elevated in some patients, but this happens only in about 50% of cases for CA-19 and 15–20% for CEA (6,9). On the other hand, CA 19-9 levels higher than 100 U/mL were associated with higher recurrence risk after surgery (10).
According to the Liver Cancer Study Group of Japan (LCSGJ), the macroscopic appearance of ICC is divided in 3 categories (11,12):
- Mass-forming subtype: nodular and exophytic masses within the liver that represent 60% to 80% of ICC cases;
- Periductal infiltrating subtype: corresponding to 15–35% of ICC, it shows diffuse and longitudinal infiltration along the biliary tree and portal tracts, resulting in biliary stricture with dilation of peripheral bile ducts;
- Intraductal growth subtype: it shows intraluminal tumor growth, forming papillary, polypoid or glandular patterns with superficial spread along the bile duct (8% to 29% of ICC).
It is often possible to distinguish these morphologic subtypes of ICC in routinely performed preoperative imaging studies, such as ultrasound, computed tomography (CT) scan and magnetic resonance imaging (MRI). Multidetector CT scan and MRI are comparable in diagnostic accuracy for radiological staging and resectability valuation. However, MRI associated with MR cholangiopancreatography offers superior anatomical visualization of bile ducts and related vascular structures (13). The use of fluorodeoxyglucose positron-emission tomography scan (FDG-PET) can also be useful for detecting regional lymph node (LN) and distant metastasis, as 80–90% of ICC will be avid. This rate seems to be higher in mass-forming subtype (85%) and lower in intraductal growth subtype (18%) (14). Some small series have shown that the use of FGD-PET may reveal occult distant metastases and change therapeutic management in 20% to 30% of patients (15,16). On the other side, when CT scan or MRI show no suspicious disease outside the liver, the use of FDG-PET is questionable (13). The role of FGD-PET in ICC diagnostic work-up is still unclear and it should be utilized selectively (17).
Endoscopic retrograde cholangiopancreatography and percutaneous transhepatic cholangiography are mainly used for therapeutic intervention, but can also collect tissue samples for pathocytological analysis. Nevertheless, the diagnostic accuracy remains low, even when associated with advanced cytologic techniques, such as digitized image analysis (DIA) or fluorescence in-situ hybridization (FISH) analysis (18,19).
According to the expert consensus statement in ICC (17), liver mass biopsy is not necessary if an ICC is suspected and a curative hepatic resection is planned. Biopsy is required before locoregional or systemic therapies are initiated in the context of unresectable disease though. If obtained, immunostaining is required to differentiate ICC from metastatic lesions and mixed hepatocellular neoplasms. If adenocarcinoma is shown in a liver mass biopsy, an occult extrahepatic primary tumor should be ruled out, unless the immunohistochemical staining panel and imaging are clearly indicative of ICC.
Staging
Until the 6th edition of American Joint Committee on Cancer (AJCC) staging system, TMN staging for ICC was associated with HCC as primary liver malignancies. In the 7th edition, a separated staging system for ICC was proposed that took into account to define the T stage the number of lesions, vascular invasion, intrahepatic metastasis and adjacent tissue invasion, but not tumor size (20). The recent 8th edition included it by considering different prognostic values for tumors ≤5 (T1a) and >5 cm (T1b) (21). This modification is justified by the better prognosis of T1aN0M0 tumors (stage IA) compared to T1bN0M0 tumors (stage IB), with 5-year overall survival (OS) of 57.8% and 44.5%, respectively. Multicentric tumors or single tumors with vascular invasion (T2) impose 5-year OS of 30.5% (T2N0M0, stage II), whereas tumors perforating the visceral peritoneum (T3) show 5-year OS of 24.4% (T3N0M0, stage IIIA). The 8th edition also recommended removal of a minimum of 6 regional LNs for adequate N stage classification and reclassified patients with regional LNs involvement from stage IVA (7th edition) to stage IIIB (8th edition). The stage IIIB thus currently encompasses tumors directing invading local extrahepatic structures (T4N0M0) and tumors with regional LNs involvement (AnyT/N1/M0), with 5-year OS of 12.4%. This modification was made because, in the absence of distant metastasis, some patients with LNs involvement may benefit from curative intent resection. Patients with distant metastasis (Any T/Any N/M1, stage IV) have dismal prognosis (5-year OS 8.4%). Only hilar, periduodenal and peripancreatic nodes are considered regional LNs.
Therapeutic options
Surgical resection
The only potentially curative treatment available for ICC is surgical resection. The prognosis is dismal without surgery, with almost null 3-years survival. Nonetheless, the outcomes of patients undergoing surgery are still unsatisfactory, with 5-year survival rate of 20–35% in some series (22-24). Compared to other liver malignancies, ICC is related to shorter survival and lower resectability rates. In this unfavorable scenario, it is paramount to establish strategies to optimize surgical results. The performance of staging laparoscopy, and lymphadenectomy, the role of neoadjuvant, adjuvant and locoregional treatments as well as better predictors of recurrence and survival are all debatable issues in ICC surgical treatment.
Preoperative evaluation
The main goal of surgical resection for ICC is to perform R0 margin negative resection with preservation of an adequate future liver remnant (FLR), which means two or more contiguous liver segments with adequate arterial and portal inflow, biliary drainage and venous outflow (17). The presence of extrahepatic disease, including involvement of LNs beyond the regional basin (for instance, celiac and the para-aortic nodes), is considered contraindication to hepatic resection (8,17). While negatively affecting outcomes, tumor size, multicentric tumors, and vascular invasion should not be considered absolute contra-indications if negative margins can be achieved. Even patients with advanced complex tumors requiring extensive hepatic resections and major vascular and biliary reconstruction should be considered for curative-intent surgery.
A FLR size of at least 20% is generally recommended for patients with no underlying liver disease. Due to the risk of post-operative hepatic insufficiency, for patients with underlying hepatic steatosis, the FLR size should be at least 30% and for those with liver cirrhosis, at least 40%, when hepatic function is still preserved (Child A patients) (25).
The performance of preoperative hepatic volumetric analysis associated with functional evaluations, such as indocyanine green clearance or 99mTc-mebrofein hepatobiliary scintigraphy, is therefore recommended before major hepatectomy for ICC (8). For patients with marginal or inadequate predicted FLR volume and function, portal vein embolization (PVE) is usually employed to cause hypertrophy of the contralateral lobe and achieve greater FLR volume (26). If properly selected and managed, patients with Child A cirrhosis or chronic liver disease as hemochromatosis may achieve similar long-term oncologic outcomes compared to patients with no underlying liver disease (27).
The FLR volume increase caused by PVE ranges between 30% and 50% after 4–8 weeks, however it also may increase the risk of drop-out by up to 30% (28,29). Associating liver partition and portal vein ligation (ALPPS) induces faster hypertrophy than PVE (more than 60% in 7 days), however it is associated with higher morbimortality (30-32). Outcomes of ALPPS for primary liver tumors are actually discouraging. In a recent review of ALPPS for ICC, López-López et al. (33) found an incidence of morbidity (Clavien’s grade III or higher) and postoperative mortality of 50% and 45.4%, respectively. The same group proposes an alternative technique, called tourniquet-ALPPS, for treatment of very large ICC (33). In stage 1, the appropriated main branch of the portal vein is ligated and a tourniquet is placed within the umbilical fissure or main portal fissure. It is then tightened enough to occlude vascular communication between lobes. No liver partition is performed. In stage 2, the tourniquet is used as an aid to the hanging maneuver and liver partition is performed by the anterior approach. In a small casuistic of 5 patients with very large ICC requiring extended right hepatectomy, postoperative mortality was 20%. Inferior vena cava resection was performed in 3 cases. PVE remains the gold-standard when FLR hypertrophy is needed, but tourniquet-ALPPS may be considered in extreme cases.
Preoperative serum albumin and total bilirubin levels can predict the risk of postoperative hepatic failure and indicate worse prognosis, when lower than 3 mg/dL and or higher than 10 mg/dL, respectively (34). Patients with obstructive jaundice caused by hilar bile duct invasion should be managed pre-operatively with percutaneous or endoscopic biliary drainage (6).
Staging laparoscopy
Staging laparoscopy can be useful in ruling out small peritoneal implants or distant LN disease. When associated with intraoperative ultrasound, it can evaluate the extent of intrahepatic disease, vascular invasion and reveal intraparenchymal metastasis. Therefore, it has a potential role in detecting unresectable disease and avoiding laparotomy, which has been reported to happen in 25–36% of patients (35,36). High-risk patients—especially those with multicentric disease, high CA 19-9, suspected peritoneal implants or major vascular invasion—benefit from staging laparoscopy. Its routine use with laparoscopic ultrasound in these cases is therefore advocated.
Lymphadenectomy
LN metastasis (LNM) is a crucial prognostic factor in ICC. Albeit controversial in the recent past, routine lymphadenectomy at time of resection for ICC is recommended by latest guidelines (9,17). LN metastasis is common in ICC, happening in around 40% of cases (23,37). Most importantly, tumor specific prognostic factors, such as lesion size, tumor centricity, margin status and vascular invasion are much less relevant in the presence of LMN, as prognosis is dictated by nodal disease. In a systematic review of cases operated between 1973 and 2012 (n=2,358), Amini et al. found that lymphadenectomy was performed in 78.5% of patients, with 45.2% incidence of LNM (38). They reported 3- and 5-year OS of 0.2% and 0% for patients with LMN compared to 55.6% and 45.1%, respectively, for patients without LMN (38).
In an international multicentric database review, compromising 1,084 patients who underwent curative-intent hepatic resection for ICC, Zhang et al. reported that only 49.9% of patients underwent concomitant lymphadenectomy (39). However, this proportion more than doubled over time, from 44.4% in 2000 to 81.5% in 2015, in both Western and Eastern centers. The median number of retrieved LNs was four and 18.8% of patients had six or more nodes evaluated. In multivariate analysis, the use of lymphadenectomy was associated with the period of time in which the operation was performed, the extent of the T stage, presence of biliary invasion and performance of major hepatectomy as well as vascular and bile duct resection. The use of lymphadenectomy was higher in Western than Eastern centers (60.4% versus 33.2%). However, tumors treated in the West were more advanced and more likely to require major hepatectomy (67.2% versus 27%). Interestingly, when these factors were controlled for in multivariate analysis, the use of lymphadenectomy was similar between centers.
Zhang et al. analyzed the SEER (Surveillance, Epidemiology, and End Results) database and identified 1,498 patients who underwent hepatic resection for ICC in the USA from 2000 to 2013 (40). In contrast to the observed in highly specialized hepatobiliary centers (39), the use of lymphadenectomy remained relatively low and did not change over time in this national sample of hospitals (2000–2004: 50.5% vs. 2005–2009: 52% vs. 2010–2013: 53.7%, P=0.636). This is worrisome, since the T-stage is not a reliable predictor of nodal status. The association of LNM with T1 ranges between 22 to 24% (T1a: 24%, T1b: 22%), with T2, from 42.9% to 55.3%, with T3, from 48% to 51.4% and with T4, from 39% to 66% (39,40).
In view of these data, the 8th AJCC revision staging system formally recommended the sampling of at least 6 regional LNs (21). This recommendation nonetheless is not evidence-based as the incidence of 6 nodes being evaluated after lymphadenectomy is low (11.4% to 18.8%) (39,40). Older series show that less than 25% of cases have at least 4 nodes retrieved (41). There is a trend, however, of more cases with ≥6 LNs over time (2000–2004: 6.9%, 2005–2009: 10.6% vs. 2009–2013: 14.4%) (40). Brauer et al. (42) analyzed the US National Cancer database in order to determine the optimal number of LNs to be retrieved. They studied 922 patients who underwent R0 resection with regional lymphadenectomy for ICC between 2004 and 2012. However, no threshold of LNs was associated with OS (42). Despite this data, the importance of lymphadenectomy as an indispensable tool for determining the precise staging of ICC remains, since it can guide the use of adjuvant therapies in case of nodal disease. Moreover, some retrospective studies have suggested a possible therapeutic benefit in reducing locoregional recurrence, but this has not been demonstrated in prospective series (43,44).
Regarding the surgical technique, the LNs along the hepatoduodenal ligament and the common hepatic artery should be removed in all cases. For ICC arising in the right hemiliver, retropancreatic and portocaval nodes must also be resected. For ICC in the left hemiliver, LNs along the lesser curvature and cardia of the stomach should be removed as well (8,17).
For patients with preoperative imaging studies showing grossly positive porta hepatis LNs, systemic therapy is recommended as the initial treatment, given the dismal prognosis of node positive disease (median survival 7–14 months) (17). Subsequent re-staging may be performed to identify patients with satisfactory response and possible candidates for surgery.
Cirrhotic patients compromise a separate group in whom lymphadenectomy should be employed with discretion, since it is related to increased risk of complications. Bagante et al. showed a complication rate of 71% in 118 cirrhotic patients, significantly higher than the 23% rate observed in non-cirrhotic patients (n=887) (45). Infectious complications were especially prevalent in cirrhotics. For these patients, preoperative criteria which identify low-risk groups for LMN are useful, such as the one proposed by Yoh et al., who showed that patients with CA 19-9 levels <37 UI/mL, peripheral ICC and no LN swelling on imaging studies have a false negative rate of only 2.3% (46).
Postoperative outcomes and predictors of prognosis
The 8th AJCC staging edition demonstrated the following stratification of 5-year OS: stage IA 57.8%, stage IB 44.5%, stage II 30.5%, stage IIIA 24.4%, stage IIIB 12.4% and stage IV 8.6% (21). With adequate patient selection, 5-year OS may reach 30–40% (23,47,48). For instance, patients with solitary ICC lesions ≤5 cm undergoing R0 resection can achieve 71% 5-year OS (49).
Mavros et al. performed a systematic review of 57 studies compromising 4,756 patients who underwent curative-intent hepatic resection for ICC (48). Most patients underwent major hepatectomy (82%) and lymphadenectomy (67%) with R0 margins (74%). Extrahepatic bile duct resection was performed in 23%. The incidence of LNM, vascular, perineural or biliary invasion was 34%, 38%, 29% and 29%, respectively. Median and 5-year recurrence free survival (RFS) ranged from 7 to 34 months and 2% to 39%, respectively. Shorter OS was associated with older age, large tumor size, multicentric tumor and satellite lesions, LMN, vascular and perineural invasion, positive surgical margins, major liver resection, extrahepatic bile duct resection, presence of cirrhosis, hepatitis B virus infection postoperative morbidity, multiple blood transfusions and poor tumor differentiation.
Besides LN positive disease, multifocal tumor remains a strong predictor of unfavorable prognosis. Compared to solitary lesions, the median survival decreases importantly: 87 vs. 19 months (50). Other notable prognostic factors include the morphological status and ICC localization within the liver. In an international multicentric study, Bagante et al. showed that patients with mass-forming or intraductal-growth ICC had 41.8% 5-year survival, while those with periductal-infiltrating subtype or combined mass-forming and periductal-infiltrating ICC presented 25.5% 5-year OS, which corresponded to a 45% increased risk of death in the long-term (51). Compared to mass-forming ICC, the intraductal-growth subtype showed higher incidence of poor differentiated tumors, lymph-vascular and perineural invasion in another study of Bagante et al. (52). However, after controlling for these variables, multivariate analysis showed similar prognosis between these two subtypes (52). Regarding ICC localization, Zhang et al. (1) showed that mass-forming ICC with hilar involvement (hICC) presented worse prognosis than peripheral mass-forming ICC (pICC) and pCCA. More aggressive tumor characteristics were found on hICC group, such as vascular invasion and LNM, as well as more extensive surgical procedures and higher complications rates (1). Interestingly, on multivariate analysis the hilar involvement was not associated with survival, which was instead dictated by biological tumor characteristics, such as T stage, nodal status and tumor differentiation (1). Similar results were also reported by Orimo et al., who showed that hICC had more aggressive biological behavior with lower survival than pICC (5 years OS: 30.4% vs. 51.1%) (53).
Increasing tumor size and tumor multifocality are related to extended hepatectomy, vascular, extrahepatic bile ducts and multivisceral resection, which translates in worse long-term outcomes. However, these factors cannot be considered absolute contra-indications for curative treatment (8). Spolverato et al. (54) compared 215 patients with solitary ICC smaller than 7 cm to 342 patients with multifocal ICC with one lesion larger than 7 cm. The advanced tumor group showed higher proportion of extended hepatectomies (30.4% vs. 16.9%), vascular invasion (38.5% vs. 22.5%), adjacent organs invasion (12.9% vs. 6.5%) and nodal metastasis (46.8% vs. 39.3%). Consequently, 5-year RFS and OS were lower (8.5% vs. 22.6% and 18.7% vs. 30.5%, respectively). Postoperative morbimortality was similar between groups. Factors associated with worse outcome in this group were more than 3 tumor nodules, nodal metastasis and poor differentiation. Bartsch et al. (55) reported their experience with 102 resected ICC, with a high proportion of vascular (27.4%) and visceral resections (34.3%), including adrenal gland, pericardium, duodenum, colon, diaphragm, hilar bifurcation, inferior vena cava, portal vein and hepatic artery. Aggressive surgical approach achieved R0 resections in 85% of cases. In-hospital mortality was 8.8%, especially after resection of 5 liver segments. The 5-year OS rate was 21%, and visceral infiltration was major predictor of worse outcomes in multivariate analysis (55). In another series from Ali et al. (56), addressing 121 major hepatectomies for ICC, major vascular resection (inferior vena cava and portal vein) was performed in 12% of cases and no difference in outcome was observed compared to patients who did not undergo vascular resection (56). Similar results were found by Reames et al. in a recent multi-institutional analysis of 1,087 patients with 12% of major vascular resections (57).
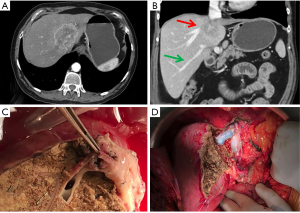
A few numbers of ex situ liver resections and auto-transplantation have been described for ICC. These extreme procedures offer opportunity for R0 resection but require the use of liver transplantation techniques, such as hypothermic liver perfusion, veno-venous bypass and total vascular exclusion (58). For ICC, the most common indication is the invasion of the confluence of three hepatic veins and retrohepatic vena cava, requiring reconstruction of inferior vena cava and reimplantation of the liver remnant into it (59). Therefore, in properly selected patients, the need of visceral or major vascular resections is not a contra-indication to attempt curative-intention surgery for ICC. Figure 1 depicts a case of large ICC invading the hepatic confluence that required ex vivo left hepatectomy with right hepatic vein reconstruction and reimplantation of the right lobe.
In fact, microscopically negative resection margins may be challenging to achieve in cases of large tumors. Albeit R0 being the goal of curative-intent resection, the width of surgical margins is important, at least for patients without nodal disease. When nodal disease is present, R1 resections is not associated with worse survival compared to R0 margins (24). Hence, oncologic outcome is dictated by nodal metastases in these cases. Nevertheless, among patients with N0 disease, R1 margins negatively impact prognosis and median survival is continuously increased by wider margins (≤1 mm: 15 months; 2–4 mm: 36 months; 5–9 mm: 57 months; ≥10 mm: 64 months) (24). Similar results were reported by Spolverato et al. in a multi-institution database of 583 patients (60). Cases with positive margins and margin width negative by 1–4, 5–9 and ≥10 mm presented 5-year OS of 13.1, 13.8, 27, 33.2 months, respectively. Margin status and width remained independently associated with recurrence and OS on multivariate analyses. Resection margins ≥10 mm are therefore desirable. Positive and narrow negative margins may be considered for adjuvant therapies, especially external beam radiation, although there is no prospective data to support their benefit (8).
A continuous growth is observed in minimally invasive liver resections over the last decades. The complexity of cases operated on with laparoscopic and robotic surgeries has also increased. For treatment of ICC, the experience is still limited (61). Some small series arising from single centers have shown the feasibility of performing laparoscopic major hepatectomy and lymphadenectomy with oncologic outcomes similar to open resection (62-64). Regarding the robotic approach, the experience is even more restricted and composed by case reports for pCCA (61). The use of minimally invasive surgery for ICC seems feasible and safe, but more studies are needed in this field.
Treatment of recurrence
Even with R0 hepatic resection, ICC recurrence remains regrettably high with 5-year rate of 53.5% to 73.4% (65-68). Recurrences are exclusively intrahepatic in about 60% of cases, exclusively extrahepatic in about 13% to 19% and both in 21% to 27% (65,68). The lungs, distal LNs and peritoneum are the most common sites of extrahepatic recurrence (23.7%; 24.7%; 16.1%, respectively) (68). The optimal cut-off value to differentiate early and late recurrence is reported to be 24 months (68). A multi-institutional analysis by Zhang et al. of 685 patients with recurrence showed early recurrence in 78.8% and late in 21.2% (68). Extrahepatic disease was more common in early recurrence cases (44.1% vs. 28.3%, respectively), whereas a higher proportion of exclusively intrahepatic disease was found in late recurrence (55.9% vs. 71.7%, respectively). Interestingly, tumor characteristics (including size, satellite lesion, LNM and microvascular invasion) and narrow surgical margins were related to early recurrence. Adjuvant chemoradiotherapy was associated with decreased risk of early recurrence. For late recurrence, liver cirrhosis was the only identifiable predictor factor. Patients with early intrahepatic disease tended to have a worse OS than those with later intrahepatic recurrence (13 vs. 24 months, respectively). This data strongly suggests that intrahepatic early recurrence is probably a recurrence of the primary tumor, while late recurrence is most likely related to de novo cancer and the background liver. On the other hand, another casuistic of 137 patients with recurrence reported that most recurrences within 24 months typically involved the liver (83%) while recurrence beyond 24 months tended to be extrahepatic (61%) (69), thus the opposite of the data found in Zhang et al.’s study (68). Further studies are needed to shed light on this issue.
Considering that intrahepatic disease is the most frequent site of recurrence, new surgical resection may improve outcome in properly selected patients, especially regarding performance status and size of FLR. In Spolverato et al. multicentric series of 563 patients (66), recurrence rate was roughly 70% (n=400). Of these, 41 patients underwent repeat hepatectomy and showed superior survival than those treated with intra-arterial therapy or systemic chemotherapy (26.1 vs. 9.6 vs. 16.8 months). However, more than a half experienced a second recurrence within median time of 11.5 months (66). In the study from Zhang et al. (68), 406 patients developed intrahepatic-only recurrences, of which 103 (25.4%) underwent further curative treatment (new surgical resection: 81 cases, ablation 22 cases). The 5-year OS rate of these patients was 63.7%, comparable to the rate found in 248 patients with no recurrence (77.1%) (68). In another study from Yoh et al., a total of 15 patients with recurrent ICC underwent re-resection (7 with intrahepatic and 8 with extrahepatic disease) (67). Patients were selected according to “macroscopically resectable disease” and clinicopathological features of the primary tumor, especially nodal status, number of lesions and time to recurrence. Only 14% of patients with recurrence were operated on. They had better 3 year-survival after recurrence (SAR) than patients who did not undergo surgery: 86.7% vs. 8.7%, median SAR time 91.6 vs. 10.4 months, respectively (67).
For cases deemed unresectable, treatment options are limited to locoregional therapy and systemic chemotherapy. The first include hepatic arterial infusion (HAI) therapy and embolization therapies including both bland embolization and transarterial embolization (TACE) with or without drug-eluting beads and yttrium-labelled selective internal radiation therapy (17). The use of radiofrequency ablation, cryotherapy and microwave ablation is less used because of common large size of ICC and increased risk of biliary complications for tumors near the hilum and pedicles (5). Locoregional therapies can also be used to treat recurrence, especially in cases without performance status for surgery. There is not prospective study comparing locoregional therapies with re-resection and thus decision must be made individually. For metastatic ICC, systemic chemotherapy most commonly compromises cisplatin and gemcitabine regimens (17). Several emerging molecularly targeted therapies are being developed based on the genomic alterations detected in ICC (70).
Neoadjuvant therapy
A possible benefit of neoadjuvant chemotherapy on outcomes for patients with ICC has not been demonstrated in prospective randomized trials. There are retrospective series and single-institution casuistic of neoadjuvant chemotherapy with and without locoregional treatments to treat occult metastasis, decrease recurrence rates and downstage initially unresectable tumors in selected patients; however, these treatments remain controversial (71). For patients with unresectable disease and good performance status, gemcitabine/cisplatin therapy may successfully downstage 25% to 50% of patients and allow surgery (72,73). A combination with ytrium90-radioembolization has also been used with promising results (74).
Adjuvant therapy
Given the high recurrence rates observed in surgically treated ICC, the use of adjuvant therapy seems useful. However, its exact role remains to be defined. The clearest indication is for patients who underwent R1 resections or had LN disease (8). In a large retrospective multicentric series (n=1,154), Reames et al. identified 347 patients who received adjuvant chemotherapy (most commonly gemcitabine-based regimen) (75). Improved OS was found in subsets of patients at high risk for recurrence, namely those with N1 disease and those with T2/T3/T4 tumors. In a systematic review and meta-analysis of 20 studies including 6,000 patients with biliary tract cancer, systemic chemotherapy associated or not with radiation showed better results than radiation alone for patients with positive margins or LNM (76). The most common regimes were gemcitabine or 5-fluorouracil (5-FU) systemic treatment and 5-FU-based radiation. Retrospective institutional data suggest that radiation may offer some benefit in decreasing recurrence risk (77,78). Adjuvant therapy may also be considered in patients with others high-risk features, such as satellitosis/multicentric tumors and poor differentiation, especially in the setting of clinical trials (17).
Liver transplantation
The use of orthotopic liver transplantation (OLT) for unresectable ICC or ICC arising in the context of liver cirrhosis have historically been poor, with 18–25% 5-year OS (79,80). Thus, it was considered a formal contra-indication for OLT in many centers. Interest was regained after a study reported that patients with PSC with incidentally discovered ICC on explanted liver had similar survival to patients without ICC (5-year OS 83%) (81). However, more recent results of many transplant centers remained poor, with 5-year OS of roughly 30% (82-84). More recent data shows that 5-year OS reaches 65% for OLT performed in cirrhotics with small solitary ICC (≤2 cm) that could not undergo resection (85). However, these results are limited to well-differentiated ICC, as moderately-differentiated tumors presented worse outcomes.
In a retrospective study comparing OLT to liver resection, Hong et al. demonstrated that patients who received neoadjuvant therapy prior to OLT experienced better 5-year OS (2). Only one prospective study about OLT for ICC is found on literature, performed by Lunsford et al., involving patients with unresectable ICC (86). Inclusion criteria include solitary tumor ≥2 cm or multifocal disease confined to the liver without radiological evidence of extrahepatic, macrovascular or LN involvement. Patients were considered eligible for protocol consideration if they had shown 6 months of disease stability or tumor regression after neoadjuvant chemotherapy. All patients were surgically staged at time of laparotomy before the transplant was performed. A total of 12 patients were enrolled. Six patients underwent transplant, 3 were on the waitlist at time of publication and 3 were not transplanted because of findings during laparotomy (multiple adhesions in 2 and resectable disease in 1). Median time from diagnosis to transplantation was 26 months. One patient died 14.5 months after OLT because of metastatic disease and 3 patients developed recurrence at a median time after OLT of 7.6 months, which translates into 5-years OS and disease-free survival of 83.3% and 50%, respectively (86).
Considering the scarcity of donors, liver transplantation for ICC, even in cases of PSC, should be considered only in very selected cases. The response to neoadjuvant therapy seems to be a crucial factor for performing OLT for ICC with good results.
Conclusions
In conclusion, ICC remains a challenging disease, with increasing incidence worldwide. As an aggressive primary liver malignancy, surgical resection is the only potentially curative treatment. The principle of the surgical approach is a margin negative hepatic resection with preservation of adequate liver remnant. Staging laparoscopy is warranted in high-risk patients to avoid unnecessary laparotomy. Regional lymphadenectomy is recommended at time of hepatectomy due to the massive impact on outcomes caused by LN metastasis. Multicentric disease, tumor size, margin status and tumor differentiation are also important prognostic factors. High-complex procedures, such as major vascular, extrahepatic bile ducts and visceral resections, ex vivo hepatectomy and autotransplantation, should be implemented in properly selected patients to achieve R0 margins. Recurrence is frustratingly high, but some patients can be selected for repeated resection. Neoadjuvant therapy may be used in initially unresectable lesions in order to downstage and allow resection. Adjuvant therapy should be considered in cases with unfavorable prognostic factors. Despite the historically poor outcomes of liver transplantation for these tumors, highly selected patients with unresectable disease, especially those with adequate response to neoadjuvant therapy, may be offered transplant within clinical trials.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Zhang XF, Bagante F, Chen Q, et al. Perioperative and long-term outcome of intrahepatic cholangiocarcinoma involving the hepatic hilus after curative-intent resection: comparison with peripheral intrahepatic cholangiocarcinoma and hilar cholangiocarcinoma. Surgery 2018;163:1114-20. [Crossref] [PubMed]
- Hong JC, Jones CM, Duffy JP, et al. Comparative analysis of resection and liver transplantation for intrahepatic and hilar cholangiocarcinoma: a 24-year experience in a single center. Arch Surg 2011;146:683-9. [Crossref] [PubMed]
- Aljiffry M, Abdulelah A, Walsh M, et al. Evidence-based approach to cholangiocarcinoma: a systematic review of the current literature J Am Coll Surg 2009;208:134-47. [Crossref] [PubMed]
- Shaib YH, Davila JA, McGlynn K, et al. Rising incidence of intrahepatic cholangiocarcinoma in the United States: a true increase? J Hepatol 2004;40:472-7. [Crossref] [PubMed]
- Dodson RM, Weiss MJ, Cosgrove D, et al. Intrahepatic cholangiocarcinoma: management options and emerging therapies. J Am Coll Surg 2013;217:736-50.e4. [Crossref] [PubMed]
- Wang K, Zhang H, Xia Y, et al. Surgical options for intrahepatic cholangiocarcinoma. Hepatobiliary Surg Nutr 2017;6:79-90. [Crossref] [PubMed]
- Palmer WC, Patel T. Are common factors involved in the pathogenesis of primary liver cancers? A meta-analysis of risk factors for intrahepatic cholangiocarcinoma. J Hepatol 2012;57:69-76. [Crossref] [PubMed]
- Squires MH. Cloyd JM1, Dillhoff M1, Schmidt C1, Pawlik TM1. Challenges of surgical management of intrahepatic cholangiocarcinoma. Expert Rev Gastroenterol Hepatol 2018;12:671-81. [Crossref] [PubMed]
- Maithel SK, Gamblin TC, Kamel I, et al. Multidisciplinary approaches to intrahepatic cholangiocarcinoma. Cancer 2013;119:3929-42. [Crossref] [PubMed]
- Tamandl D, Herberger B, Gruenberger B, et al. Influence of hepatic resection margin on recurrence and survival in intrahepatic cholangiocarcinoma. Ann Surg Oncol 2008;15:2787-94. [Crossref] [PubMed]
- Yamasaki S. Intrahepatic cholangiocarcinoma: macroscopic type and stage classification. J Hepatobiliary Pancreat Surg 2003;10:288-91. [Crossref] [PubMed]
- Nakanuma Y, Sato Y, Harada K, et al. Pathological classification of intrahepatic cholangiocarcinoma based on a new concept. World J Hepatol 2010;2:419-27. [Crossref] [PubMed]
- Chong YS, Kim YK, Lee MW, et al. Differentiating mass-forming intrahepatic cholangiocarcinoma from atypical hepatocellular carcinoma using gadoxetic acid-enhanced MRI. Clin Radiol 2012;67:766-73. [Crossref] [PubMed]
- Anderson CD, Rice MH, Pinson CW, et al. Fluorodeoxyglucose PET imaging in the evaluation of gallbladder carcinoma and cholangiocarcinoma. J Gastrointest Surg 2004;8:90-7. [Crossref] [PubMed]
- Kim YJ, Yun M, Lee WJ, et al. Usefulness of 18F-FDG PET in intrahepatic cholangiocarcinoma. Eur J Nucl Med Mol Imaging 2003;30:1467-72. [Crossref] [PubMed]
- Corvera CU, Blumgart LH, Akhurst T, et al. 18F-fluorodeoxyglucose positron emission tomography influences management decisions in patients with biliary cancer. J Am Coll Surg 2008;206:57-65. [Crossref] [PubMed]
- Weber SM, Ribero D, O'Reilly EM, et al. Intrahepatic cholangiocarcinoma: expert consensus statement. HPB (Oxford) 2015;17:669-80. [Crossref] [PubMed]
- Baron TH, Harewood GC, Rumalla A, et al. A prospective comparison of digital image analysis and routine cytology for the identification of malignancy in biliary tract strictures. Clin Gastroenterol Hepatol 2004;2:214-9. [Crossref] [PubMed]
- Kipp BR, Stadheim LM, Halling SA, et al. A comparison of routine cytology and fluorescence in situ hybridization for the detection of malignant bile duct strictures. Am J Gastroenterol 2004;99:1675-81. [Crossref] [PubMed]
- Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol 2010;17:1471-4.
- Amin MB, Edge S, Greene F, et al. AJCC Cancer Staging Manual. 2017. Available online: https://www.springer.com/la/book/9783319406176
- Wang Y, Li J, Xia Y, et al. Prognostic nomogram for intrahepatic cholangiocarcinoma after partial hepatectomy. J Clin Oncol 2013;31:1188-95. [Crossref] [PubMed]
- Ribero D, Pinna AD, Guglielmi A, et al. Surgical Approach for Long-term Survival of Patients With Intrahepatic Cholangiocarcinoma: A Multi-institutional Analysis of 434 Patients. Arch Surg 2012;147:1107-13. [Crossref] [PubMed]
- Farges O, Fuks D, Boleslawski E, et al. Influence of surgical margins on outcome in patients with intrahepatic cholangiocarcinoma: a multicenter study by the AFC-IHCC-2009 study group. Ann Surg 2011;254:824-29. [Crossref] [PubMed]
- Thirunavukarasu P, Aloia TA. Preoperative Assessment and Optimization of the Future Liver Remnant. Surg Clin North Am 2016;96:197-205. [Crossref] [PubMed]
- Nagino M, Kamiya J, Nishio H, et al. Two hundred forty consecutive portal vein embolizations before extended hepatectomy for biliary cancer: surgical outcome and long-term follow-up. Ann Surg 2006;243:364-72. [Crossref] [PubMed]
- Sulpice L, Rayar M, Boucher E, et al. Intrahepatic cholangiocarcinoma: impact of genetic hemochromatosis on outcome and overall survival after surgical resection. J Surg Res 2013;180:56-61. [Crossref] [PubMed]
- Shindoh J, Vauthey JN, Zimmitti G, et al. Analysis of the efficacy of portal vein embolization for patients with extensive liver malignancy and very low future liver remnant volume, including a comparison with the associating liver partition with portal vein ligation for staged hepatectomy approach. J Am Coll Surg 2013;217:126-33. [Crossref] [PubMed]
- Robles R, Marín C, Lopez-Conesa A, et al. Comparative study of right portal vein ligation versus embolisation for induction of hypertrophy in two-stage hepatectomy for multiple bilateral colorectal liver metastases. Eur J Surg Oncol 2012;38:586-93. [Crossref] [PubMed]
- Tanaka K, Matsuo K, Murakami T, et al. Associating liver partition and portal vein ligation for staged hepatectomy (ALPPS): short-term outcome, functional changes in the future liver remnant, and tumor growth activity. Eur J Surg Oncol 2015;41:506-12. [Crossref] [PubMed]
- Herman P, Krüger JA, Perini MV, et al. High Mortality Rates After ALPPS: the Devil Is the Indication. J Gastrointest Cancer 2015;46:190-4. [Crossref] [PubMed]
- Aloia TA. Associating Liver Partition and Portal Vein Ligation for Staged Hepatectomy: Portal Vein Embolization Should Remain the Gold Standard. JAMA Surg 2015;150:927-8. [Crossref] [PubMed]
- López-López V, Robles-Campos R, Brusadin R, et al. Tourniquet-ALPPS is a promising treatment for very large hepatocellular carcinoma and intrahepatic cholangiocarcinoma. Oncotarget 2018;9:28267-80. [Crossref] [PubMed]
- Mosconi S, Beretta GD, Labianca R, et al. Cholangiocarcinoma. Crit Rev Oncol Hematol 2009;69:259-70. [Crossref] [PubMed]
- Goere D, Wagholikar GD, Pessaux P, et al. Utility of staging laparoscopy in subsets of biliary cancers: laparoscopy is a powerful diagnostic tool in patients with intrahepatic and gallbladder carcinoma. Surg Endosc 2006;20:721-5. [Crossref] [PubMed]
- D'Angelica M, Fong Y, Weber S, et al. The role of staging laparoscopy in hepatobiliary malignancy: prospective analysis of 401 cases Ann Surg Oncol 2003;10:183-9. [Crossref] [PubMed]
- Luo X, Yuan L, Wang Y, et al. Survival outcomes and prognostic factors of surgical therapy for all potentially resectable intrahepatic cholangiocarcinoma: a large single-center cohort study. J Gastrointest Surg 2014;18:562-72. [Crossref] [PubMed]
- Amini N, Ejaz A, Spolverato G, et al. Management of lymph nodes during resection of hepatocellular carcinoma and intrahepatic cholangiocarcinoma: a systematic review. J Gastrointest Surg 2014;18:2136-48. [Crossref] [PubMed]
- Zhang XF, Chakedis J, Bagante F, et al. Trends in use of lymphadenectomy in surgery with curative intent for intrahepatic cholangiocarcinoma. Br J Surg 2018;105:857-66. [Crossref] [PubMed]
- Zhang XF, Chen Q, Kimbrough CW, et al. Lymphadenectomy for Intrahepatic Cholangiocarcinoma: Has Nodal Evaluation Been Increasingly Adopted by Surgeons over Time?A National Database Analysis. J Gastrointest Surg 2018;22:668-75. [Crossref] [PubMed]
- Vitale A, Moustafa M, Spolverato G, et al. Defining the possible therapeutic benefit of lymphadenectomy among patients undergoing hepatic resection for intrahepatic cholangiocarcinoma. J Surg Oncol 2016;113:685-91. [Crossref] [PubMed]
- Brauer DG, Fields RC, Tan BR Jr, et al. Optimal extent of surgical and pathologic lymph node evaluation for resected intrahepatic cholangiocarcinoma. HPB (Oxford) 2018;20:470-6. [Crossref] [PubMed]
- Nakagawa T, Kamiyama T, Kurauchi N, et al. Number of lymph node metastases is a significant prognostic factor in intrahepatic cholangiocarcinoma. World J Surg 2005;29:728-33. [Crossref] [PubMed]
- Shimada K, Sano T, Nara S, et al. Therapeutic value of lymph node dissection during hepatectomy in patients with intrahepatic cholangiocellular carcinoma with negative lymph node involvement. Surgery 2009;145:411-6. [Crossref] [PubMed]
- Bagante F, Spolverato G, Weiss M, et al. Surgical Management of Intrahepatic Cholangiocarcinoma in Patients with Cirrhosis: Impact of Lymphadenectomy on Peri-Operative Outcomes. World J Surg 2018;42:2551-60. [Crossref] [PubMed]
- Yoh T, Hatano E, Seo S, et al. Preoperative criterion identifying a low-risk group for lymph node metastasis in intrahepatic cholangiocarcinoma. J Hepatobiliary Pancreat Sci 2018;25:299-307. [Crossref] [PubMed]
- de Jong MC, Nathan H, Sotiropoulos GC, et al. Intrahepatic cholangiocarcinoma: an international multi-institutional analysis of prognostic factors and lymph node assessment. J Clin Oncol 2011;29:3140-5. [Crossref] [PubMed]
- Mavros MN, Economopoulos KP, Alexiou VG, et al. Treatment and Prognosis for Patients With Intrahepatic Cholangiocarcinoma: Systematic Review and Meta-analysis. JAMA Surg 2014;149:565-74. [Crossref] [PubMed]
- Tarchi P, Tabrizian P, Prigoff J, et al. Outcomes of resection for solitary ≤5 cm intrahepatic cholangiocarcinoma. Surgery 2018;163:698-702. [Crossref] [PubMed]
- Endo I, Gonen M, Yopp AC, et al. Intrahepatic cholangiocarcinoma: rising frequency, improved survival, and determinants of outcome after resection. Ann Surg 2008;248:84-96. [Crossref] [PubMed]
- Bagante F, Spolverato G, Weiss M, et al. Impact of Morphological Status on Long-Term Outcome Among Patients Undergoing Liver Surgery for Intrahepatic Cholangiocarcinoma. Ann Surg Oncol 2017;24:2491-501. [Crossref] [PubMed]
- Bagante F, Weiss M, Alexandrescu S, et al. Long-term outcomes of patients with intraductal growth sub-type of intrahepatic cholangiocarcinoma. HPB (Oxford) 2018. [Epub ahead of print].
- Orimo T, Kamiyama T, Mitsuhashi T, et al. Impact of tumor localization on the outcomes of surgery for an intrahepatic cholangiocarcinoma. J Gastroenterol 2018. [Epub ahead of print]. [Crossref] [PubMed]
- Spolverato G, Kim Y, Alexandrescu S, et al. Is Hepatic Resection for Large or Multifocal Intrahepatic Cholangiocarcinoma Justified? Results from a Multi-Institutional Collaboration. Ann Surg Oncol 2015;22:2218-25. [Crossref] [PubMed]
- Bartsch F, Baumgart J, Hoppe-Lotichius M, et al. Visceral infiltration of intrahepatic cholangiocarcinoma is most prognostic after curative resection - Retrospective cohort study of 102 consecutive liver resections from a single center. Int J Surg 2018;55:193-200. [Crossref] [PubMed]
- Ali SM, Clark CJ, Zaydfudim VM, et al. Role of major vascular resection in patients with intrahepatic cholangiocarcinoma. Ann Surg Oncol 2013;20:2023-8. [Crossref] [PubMed]
- Reames BN, Ejaz A, Koerkamp BG, et al. Impact of major vascular resection on outcomes and survival in patients with intrahepatic cholangiocarcinoma: A multi-institutional analysis. J Surg Oncol 2017;116:133-9. [Crossref] [PubMed]
- Baker MA, Maley WR, Needleman L, et al. Ex vivo resection of hepatic neoplasia and autotransplantation: a case report and review of the literature. J Gastrointest Surg 2015;19:1169-76. [Crossref] [PubMed]
- Vicente E, Quijano Y, Ielpo B, et al. Ex Situ Hepatectomy and Liver Autotransplantation for Cholangiocarcinoma. Ann Surg Oncol 2017;24:3990. [Crossref] [PubMed]
- Spolverato G, Yakoob MY, Kim Y, et al. The Impact of Surgical Margin Status on Long-Term Outcome After Resection for Intrahepatic Cholangiocarcinoma. Ann Surg Oncol 2015;22:4020-8. [Crossref] [PubMed]
- Levi Sandri GB, Spoletini G, Mascianà G, et al. The role of minimally invasive surgery in the treatment of cholangiocarcinoma. Eur J Surg Oncol 2017;43:1617-21. [Crossref] [PubMed]
- Abu Hilal M, Badran A, Di Fabio F, et al. Pure laparoscopic en bloc left hemihepatectomy and caudate lobe resection in patients with intrahepatic cholangiocarcinoma. J Laparoendosc Adv Surg Tech A 2011;21:845-9. [Crossref] [PubMed]
- Lee W, Park JH, Kim JY, et al. Comparison of perioperative and oncologic outcomes between open and laparoscopic liver resection for intrahepatic cholangiocarcinoma. Surg Endosc 2016;30:4835-40. [Crossref] [PubMed]
- Ratti F, Cipriani F, Ariotti R, et al. Laparoscopic major hepatectomies: current trends and indications. A comparison with the open technique. Updates Surg 2015;67:157-67. [Crossref] [PubMed]
- Hyder O, Hatzaras I, Sotiropoulos GC, et al. Recurrence after operative management of intrahepatic cholangiocarcinoma. Surgery 2013;153:811-8. [Crossref] [PubMed]
- Spolverato G, Kim Y, Alexandrescu S, et al. Management and Outcomes of Patients with Recurrent Intrahepatic Cholangiocarcinoma Following Previous Curative-Intent Surgical Resection. Ann Surg Oncol 2016;23:235-43. [Crossref] [PubMed]
- Yoh T, Hatano E, Seo S, et al. Long-Term Survival of Recurrent Intrahepatic Cholangiocarcinoma: The Impact and Selection of Repeat Surgery. World J Surg 2018;42:1848-56. [Crossref] [PubMed]
- Zhang XF, Beal EW, Bagante F, et al. Early versus late recurrence of intrahepatic cholangiocarcinoma after resection with curative intent. Br J Surg 2018;105:848-56. [Crossref] [PubMed]
- Doussot A, Gonen M, Wiggers JK, et al. Recurrence Patterns and Disease-Free Survival after Resection of Intrahepatic Cholangiocarcinoma: Preoperative and Postoperative Prognostic Models. J Am Coll Surg 2016;223:493-505.e2. [Crossref] [PubMed]
- Bupathi M, Ahn DH, Bekaii-Saab T. Therapeutic options for intrahepatic cholangiocarcinoma. Hepatobiliary Surg Nutr 2017;6:91-100. [Crossref] [PubMed]
- Buettner S, van Vugt JL, IJzermans JN, et al. Intrahepatic cholangiocarcinoma: current perspectives. Onco Targets Ther 2017;10:1131-42. [Crossref] [PubMed]
- Kato A, Shimizu H, Ohtsuka M, et al. Downsizing Chemotherapy for Initially Unresectable Locally Advanced Biliary Tract Cancer Patients Treated with Gemcitabine Plus Cisplatin Combination Therapy Followed by Radical Surgery. Ann Surg Oncol 2015;22 Suppl 3:S1093-9. [Crossref] [PubMed]
- Le Roy B, Gelli M, Pittau G, et al. Neoadjuvant chemotherapy for initially unresectable intrahepatic cholangiocarcinoma. Br J Surg 2018;105:839-47. [Crossref] [PubMed]
- Rayar M, Sulpice L, Edeline J, et al. Intra-arterial yttrium-90 radioembolization combined with systemic chemotherapy is a promising method for downstaging unresectable huge intrahepatic cholangiocarcinoma to surgical treatment. Ann Surg Oncol 2015;22:3102-8. [Crossref] [PubMed]
- Reames BN, Bagante F, Ejaz A, et al. Impact of adjuvant chemotherapy on survival in patients with intrahepatic cholangiocarcinoma: a multi-institutional analysis. HPB (Oxford) 2017;19:901-9. [Crossref] [PubMed]
- Horgan AM, Amir E, Walter T, et al. Adjuvant therapy in the treatment of biliary tract cancer: a systematic review and meta-analysis. J Clin Oncol 2012;30:1934-40. [Crossref] [PubMed]
- Jia AY, Wu JX, Zhao YT, et al. Intensity-modulated radiotherapy following null-margin resection is associated with improved survival in the treatment of intrahepatic cholangiocarcinoma. J Gastrointest Oncol 2015;6:126-33. [PubMed]
- Song S, Kim K, Chie EK, et al. Locoregional recurrence after curative intent resection for intrahepatic cholangiocarcinoma: implications for adjuvant radiotherapy. Clin Transl Oncol 2015;17:825-9. [Crossref] [PubMed]
- Goldstein RM, Stone M, Tillery GW, et al. Is liver transplantation indicated for cholangiocarcinoma? Am J Surg 1993;166:768-71; discussion 771-2. [Crossref] [PubMed]
- Pichlmayr R, Weimann A, Oldhafer KJ, et al. Role of liver transplantation in the treatment of unresectable liver cancer. World J Surg 1995;19:807-13. [Crossref] [PubMed]
- Goss JA, Shackleton CR, Farmer DG, et al. Orthotopic liver transplantation for primary sclerosing cholangitis. A 12-year single center experience. Ann Surg 1997;225:472-81. [Crossref] [PubMed]
- Meyer CG, Penn I, James L. Liver transplantation for cholangiocarcinoma: results in 207 patients. Transplantation 2000;69:1633-7. [Crossref] [PubMed]
- Robles R, Figueras J, Turrión VS, et al. Spanish experience in liver transplantation for hilar and peripheral cholangiocarcinoma. Ann Surg 2004;239:265-71. [Crossref] [PubMed]
- Brandsaeter B, Isoniemi H, Broomé U, et al. Liver transplantation for primary sclerosing cholangitis; predictors and consequences of hepatobiliary malignancy. J Hepatol 2004;40:815-22. [Crossref] [PubMed]
- Sapisochin G, Facciuto M, Rubbia-Brandt L, et al. Liver transplantation for "very early" intrahepatic cholangiocarcinoma: International retrospective study supporting a prospective assessment. Hepatology 2016;64:1178-88. [Crossref] [PubMed]
- Lunsford KE, Javle M, Heyne K, et al. Liver transplantation for locally advanced intrahepatic cholangiocarcinoma treated with neoadjuvant therapy: a prospective case-series. Lancet Gastroenterol Hepatol 2018;3:337-48. [Crossref] [PubMed]
Cite this article as: Waisberg DR, Pinheiro RS, Nacif LS, Rocha-Santos V, Martino RB, Arantes RM, Ducatti L, Lai Q, Andraus W, D’Albuquerque LC. Resection for intrahepatic cholangiocellular cancer: new advances. Transl Gastroenterol Hepatol 2018;3:60.


