Clinical trends and effects on quality metrics for surgical gastroesophageal cancer care
Introduction
Gastric cancer (GC) remains a formidable health care challenge worldwide due to some settings of high incidence and mortality, and because of the complexity of specific treatment options required for best care. In the U.S., the incidence of gastric adenocarcinoma (GAC) has continuously declined, while that of adenocarcinomas of the esophagus and gastroesophageal junction (GEJ) has not followed this trend (1). For either process, long-term survival remains poor despite recent improvements, as many patients afflicted are still diagnosed with advanced-stage disease, and as available multimodal treatments are still limited in efficacy (2). Gastrointestinal stromal tumors (GISTs) represent a different clinical spectrum of malignant behavior that is being increasingly recognized (3). For all these conditions, surgical therapy in form of either gastric or gastroesophageal resection remains at the center of curative outcomes for mid-stage disease. Due to the relatively low prevalence of GC in the U.S., most surgeons tend to encounter these disease entities rarely, and specialty expertise remains sparse (4). In addition, greater comorbidity and inferior survival outcomes have been observed in this setting compared to Asian results (5). Characteristic for the U.S. system is that reported findings and outcomes differ at times widely between single, large centers of excellence, smaller case numbers in tertiary care settings, and population-based data (6-9). Accordingly, a link between high-volume gastrectomy setting and superior outcomes has been identified for operative mortality and for long-term survival, although low-volume settings do not preclude high quality treatment results per se, and therefore volume cannot be considered a goal but at best a surrogate for therapeutic quality (10,11). More recently, the Centers for Medicare and Medicaid Services’ (CMS) Hospital Compare star rating system, based on self-reported hospital performance regarding clinical outcomes, patient experience, care effectiveness, care timeliness, and efficient use of medical imaging, has been associated with outcomes including readmissions and length of stay (LOS), independent from hospital volume (12); however, specific therapeutic metrics that can be used as practice standards are not represented. How then should standards for surgical GC care that carry more universal validity be set, and how should adherence to therapeutic quality standards be measured?
In this context the value of care, primarily to patients but also to payers and other stakeholders, carries great significance. Aside from the growing mandate to limit costs of care, actual quality aspects of cancer care have become increasingly recognized as being important. However, how to measure relevant quality aspects remains a formidable challenge in general, and this extends specifically into operative cancer management such as for GC. Currently, quality metrics as mandated by payers or regulatory agencies often address process-linked, “measurable” aspects that arguably carry little meaning for true “quality” of care (13). Day-to-day healthcare may generate more emphasis on whether an operation has been done, compared to how it has been planned, performed or supported. Aspects such as appropriateness of the indication in general, or the timing of operative care within the multidisciplinary therapy spectrum are less commonly traced. On the other hand, general surgical and oncologic principles that impact upon quality of care are widely accepted, including quality of staging, proper multimodality treatment planning, defining therapeutic intent, completeness of resection (including margin-negativity rate), minimizing treatment-associated risks (through spleen preservation, avoidance of blood transfusions), and steps for optimizing recovery and early outcomes as reflected by low postoperative complication rates and limited length of hospital stay (14,15). The current study was undertaken to examine clinical patterns of GC treatment within a gastrectomy experience in an academic surgical oncology practice longitudinally, and to evaluate potential metrics for therapeutic quality components as outlined within the identified spectrum of care.
Methods
The analysis examined consecutive patients undergoing gastric resection in a U.S.-based single surgical oncologist’s practice experience over 15 years, with the intent to identify clinical spectrum changes or trends of potential significance, and to assess parameters with possible relevance to surgical quality performance within this clinical spectrum. Data on patient demographics, clinicopathologic factors, operative treatment aspects and postoperative outcomes were prospectively recorded. The American Society of Anesthesiologists’ (ASA) classification was used for operative risk assignments. Underlying diagnoses were categorized based on histopathologic analyses of the resected specimens. Most patients had malignant or premalignant neoplastic conditions; however, gastrectomies performed for non-neoplastic processes remained included to support complete evaluation of non-cancer-specific quality metrics. Malignant tumors in this series originated from or involved gastric mural components between the GEJ and the pylorus; esophageal adenocarcinomas with an epicenter above the GEJ were excluded. For characterization of GEJ cancers, the classification of Siewert was utilized. TNM staging was performed for adenocarcinomas of the stomach or GEJ using 7th edition AJCC criteria.
Intraoperative therapeutic components, postoperative findings and outcomes were examined based on their putative relationship to surgical quality performance. These parameters included intraoperative blood loss, transfusions, completeness of resection (R category), spleen preservation, lymph node (LN) counts, postoperative morbidity and LOS. Transfusions included intra- and post-operative packed red blood cell (PRBC) transfusions administered irrespective of preoperative blood counts or intraoperative blood loss. LN counts were reviewed for total LNs examined, number of positive LNs identified histopathologically, and number of negative LNs; LN count analyses were restricted to patients with an indication for extended LN dissection (ELND), thus excluding patients with GISTs, benign diagnoses or merely noncurative gastrectomy for palliation of GC-related symptoms. For patients with GAC undergoing curative-intent resection, the percent of cases with at least 15 or more LNs examined was assessed, based on common staging conventions; in addition, cut-points of >15 LNs (i.e., 16 or more), as formulated within the AJCC staging criteria, and of 20 or more total LNs were examined. Postoperative complications were charted prospectively and graded according to the classification of Clavien-Dindo; grade 1 and 2 events were defined as minor, grade 3 through 5 events as major morbidity. In case of multiple complications, the highest-grade event was chosen. Any complications or lethal events reflect occurrences during the longer time interval of either in-hospital stay or 30 days postoperatively.
Clinical trends and spectrum changes were examined through dividing the cohort by three equal time intervals, each encompassing 5 years. Data are presented via descriptive statistics. Interval comparisons were made through contingency chi square analysis for categorical data, and analysis of variance (ANOVA) or Kruskal-Wallis tests for continuous data as appropriate based on data distribution. Significance of differences was accepted at P≤0.05. The StatView (SAS, Cary, NC, USA) software package was chosen for statistical analysis.
Results
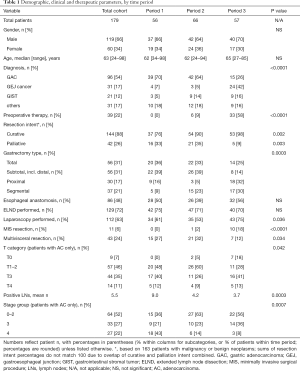
The cohort for this analysis consisted of 179 patients who had undergone gastric resection. Of these, 119 were male and 60 female, with a median age of 63 years (range, 24–98 years). Diagnoses included 96 GACs, 31 GE junction cancers, 21 GISTs, and 31 other conditions requiring some form of gastrectomy. Among the latter group, there were three neuroendocrine tumors, two soft tissue sarcomas, two nerve sheath tumors, three other benign neoplasms, five cases of isolated gastric involvement of other malignant neoplasms, and 16 cases with non-neoplastic gastric conditions. When evaluating trends over three consecutive time intervals, patient gender and age did not show obvious differences over time, while the distribution of diagnoses did: GEJ cancers became more prevalent over time, while the number of GACs operated upon decreased significantly (Table 1). Preoperative therapy, either in form of chemotherapy or chemoradiation, was given in 22% of patients overall, trending from 0% to 58% over time (P<0.0001).
Full table
Operative risk categories remained stable over time, with 60% of patients carrying ASA class 3, 31% class 2, 7% class 4 and 2% class 1 assignments. Five percent of gastrectomies were emergency procedures, while 95% were elective; 13% of resections in the first period were emergent, compared to 3% in the second and none in the last period (P=0.006). The preoperative intent among patients with a malignant or neoplastic condition (n=163) shifted over time as well, with an increase in the subset of patients operated upon with curative intent from 76% initially to 98%, and a decrease in palliation intent from 33% to 9% (Table 1). A diagnostic laparoscopy prior to definitive resection was performed in 63% of all patients (67% of cancer patients, and 74% of cancer patients with curative intent, P<0.0001), usually during the same procedure. There were only 11 minimally invasive surgical (MIS) procedures, all but one in the most recent period. An abdominal incision only was utilized for 95% of cases, compared to a thoracoabdominal incision in 5%. Resections included 56 total, 30 subtotal, 26 distal and 37 segmental gastrectomies, in addition to 30 proximal gastrectomies (or esophagogastrectomies for Siewert type 1/2 GEJ cancers). Additional operative components were ELND in 72%, splenectomy in 9%, multivisceral resection in 24%, and feeding jejunostomy tube placement in 65% of cases. Reconstructive techniques included an esophageal anastomosis in 48%, and specifically employed jejunal Roux-Y reconstruction (n=106, 59%), primary esophagogastrostomy with or without gastric pullup (n=20, 11%), Merendino small bowel interposition (n=6, 3%), Billroth-2 gastrojejunostomy (n=16, 9%) or primary gastric closure (n=31, 17%). Total operative time (mean: 5.4 h, P=0.004) and intravenous fluid administration (mean: 5,160 mL, P=0.001) showed differences but no trends between time periods.
Characteristics for operative and pathologic variables throughout the time periods are also listed in Table 1. No differences were seen for esophageal anastomosis or ELND frequencies. Time-dependent differences but no trends between the three time periods were observed for use of laparoscopy and multivisceral resections. Significant differences with directional trends between time periods were noticed for preoperative therapy, gastrectomy type, MIS resections, the T0 staging category for adenocarcinomas, positive LN number, and adenocarcinoma stage groups 3 (increase from 20% to 37%) and 4 (decrease from 44% to 8%); in addition, reconstructive techniques differed (P=0.0009), with trends for Roux-Y (71% to 40%), small bowel interposition (2% to 5%) and primary closure (7% to 26%).
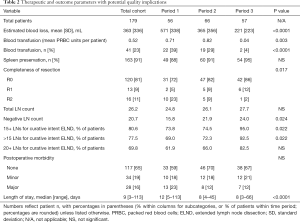
Additional variables as possible metrics for quality of care are listed in Table 2. Significant trends over time were detected for intraoperative blood loss, transfusion rates, and R categories. The overall R0 rate in 149 patients with malignancy was 81%, while among 112 cancer patients with curative resection intent and no palliative indication, a R0 resection was performed in 104 (93%). In 37 patients with malignancy and palliation needs, a R0 resection was still achieved in 43% (R1: 16%, R2: 41%). The spleen preservation rate change from 88% to 95% did not reach significance. While total LN count differences were also not significant (overall mean: 26.2), number of negative LNs and the percentage of reports with at least 15 or more than 15 LNs showed obvious trends. Postoperative morbidity consisted of minor morbidity in 19% and major morbidity in 16% of cases, the latter including eight deaths (4%); lethal events occurred in 3.3% of cancer patients without palliation needs, in 4.8% of palliative cancer resections, and in 15.4% of patients with “benign” (non-neoplastic) diagnoses. Decreasing trends in major morbidity or lethal events failed to reach significance. Finally, the postoperative LOS decreased from a median of 12 to 8 days; it also demonstrated obvious differences based on complexity of resection and postoperative morbidity (Figure 1). Other variables with impact on longer LOS included emergency resection, splenectomy, transfusion and reconstruction in form of esophagogastric anastomoses or interposition techniques (all at P<0.001).
Full table

Discussion
What represents quality in a complex clinical context, and how should it be measured? It is not by chance that metrics currently in place to assess surgical quality performance are typically reflective of process-related aspects, such as preoperative antibiotic administration, postoperative urinary catheter removal or initiation of thromboembolic prophylaxis (13). While these aspects undoubtedly carry some general importance, they are likely falling short to address domains that carry greater relevance to direct disease-specific patient benefits, appropriateness of care, and achieving best meaningful long-term outcomes. Approaching quality as an essential element to optimizing overall value would seem to benefit from the understanding that patient beneficence has to be the priority, and metrics considered most appropriate would need to reflect this aspect comprehensively and adequately representing the breadth of care spectrum (13). Equally, incentives or penalties to promote “quality” of care would need to recognize the complexity of components surrounding patient benefits, rather than relying on few selected and possibly incomplete metrics. Creating value for other stakeholders, or minimizing costs to payers and society are additional important practice components in the effort to optimize quality and value of care. How, then, should metrics be selected that are therapeutically relevant and reflect quality of upper GI cancer care delivered appropriately?
In this series, an effort was made to analyze components of operative and postoperative care as well as early outcomes for patient undergoing gastrectomy in a U.S.-based oncology practice setting. The data show a considerable variability of clinical indications and therapies, even in this relatively short time span of 15 years. While some of the changes or trends observed may well represent special practice referral patterns that are not entirely reflective of general trends, some changes nevertheless are sensible given the evolution of GC care in the past two decades: there is a significant shift away from distal GC to more GEJ cancers, and there are currently more noninvasive palliation options not requiring operative intervention any longer (14); additionally, there is a shift towards preoperative chemotherapy or chemoradiation for mid-stage gastric and GEJ cancers that qualify for curative intent treatment (16). Finally, there are changes in GIST resections consistent with an increasing recognition of GISTs representing a spectrum of neoplasms with unique biologic behavior and treatment response for which resective indications have become more refined (3,17). Under these circumstances, it appears to be a worthwhile exercise to analyze how possible quality metrics for oncologic and surgical care have fared, and whether any specific trends over time can be elucidated.
The data reveal time-dependent variations in diagnosis, a relative increase in curative-intent resections, more proximal resections, more MIS procedures, a significantly grown proportion of patients receiving preoperative therapy, and, likely related to this, fewer positive LNs and a shift towards earlier stages. Importantly, the operative complexity appears to have remained unchanged, based on consistent multivisceral resection needs, lymphadenectomy indications or esophageal anastomoses performed. Given these clinical shifts, operative blood loss and blood transfusion needs seemed to have followed a reducing trend. The higher rate of R0 resections is likely impacted by reduced palliation needs and utilization of preoperative therapies, but may also speak favorably in light of an increased number of proximal resections. In addition, R1 results are also influenced by intraoperative peritoneal washing cytology results that were routinely obtained in the first time period, and selectively but in every case of obvious or suspected serosal involvement thereafter. In contrast, R2 resections resulted almost exclusively from settings of palliation needs. In addition to care aspects where changes have been observed over time, more telling insight can perhaps be retrieved where consistent results rather than “trends” have been identified; these included total LN counts, spleen preservation rate or postoperative morbidity. For the former two, a consistency of practice and the observed results have been intended due to a perceived oncologic importance (18-20). Total or negative LN counts have been associated with superior survival results, although a direct causation remains unproven in the era of multimodality treatment options for GC (21-23). A slight increase in negative LN counts over time likely reflects preoperative therapy impact more than changes in dissection extent or pathologic examination. Overall, the number of LN obtained and the ability to properly stage patients based on a minimum LN number seem to compare favorably with other published contemporary results from North American centers: the median LN count in a recent National Cancer Database report has been 2 (24), while in academic centers represented in the US Gastric Cancer Collaborative it was 16 (25). The percentage of gastrectomy specimens with 15 or more LNs examined has been reported as 42% in the California Cancer Registry (26) and as 51% in the before entioned academic collaborative (25), compared to 81% overall in the current series, and 95% for the last time period. The trend in reduced LOS is reflective of shorter hospital durations for other surgical oncologic procedures, not just gastroesophageal resections (27,28); this is noteworthy, given a high prevalence of elderly patients and a constant morbidity rate, which both impact on the length of hospitalization (29). Spleen preservation rate, blood loss estimates and transfusion requirements in this series resemble closely those reported for curative R0 resections by Squires et al. based on combined academic institution data (30).
This series primarily compiles operative and early outcome aspects, and cannot and does not intend to make general claims on other aspects relevant to quality of care since it represents a single surgeon’s experience. Some parameters are not reported since they were either not prospectively or completely recorded, such as details of multidisciplinary treatment planning, costs of care or patient-reported aspects such as quality of life. Others remain challenging to define, e.g., appropriateness of care, especially gastrectomy indications outside the intention to cure a malignant process. For many potential therapeutic quality metrics, a key challenge is that clinical significance and impact remain arguable, as direct causative links to outcome differences are frequently not established. An example for this is the preservation of a duodenal passage after gastrectomy that while sensibly desirable has not been shown to lead to significant measurable patient benefits (31). The appropriateness of some metrics may also change, such as total LN counts for resected rectal cancer that are difficult to associate to better overall survival in light of preoperative chemoradiation (32); this particular aspect may carry relevance to GC in the setting of preoperative chemoradiation or perioperative chemotherapy, too.
How, then, should the discussion on quality metrics for surgical GC care be directed? A process that establishes consensus on which metrics are worthy of more universal adoption based on creating patient benefit and generating value to other stakeholders appears desirable. Efforts to develop enhanced postoperative recovery pathways can serve as example for such aspects of perioperative care, although their benefits over standard practice will still remain debatable unless impacted outcomes are measured and found to be benefited (15,33). Adherence to standardized practice pathways is thus not proposed to be used as surrogate for quality care, but rather measurement of relevant outcomes for patient-centered care should drive this process (34). An example for selection of relevant metrics and establishing benchmarks is the National Quality Forum colon cancer project (35); a similar forum, supported or endorsed by specialty professional societies and other stakeholders could be created for GC care. In addition to selecting therapeutic and outcome metrics, it could recommend which patient-reported parameters are to be chosen, since these are rarely studied prospectively and tend to vary widely (36,37). Although some initial efforts to nationally standardize quality metrics for cancer care tend to select parameters at what some would consider minimum-requirement thresholds, a beneficial clinical aspect has become traceable (35,38,39).
Presenting the results of this study is intended to make a contribution to the discussion on how some therapeutic and outcome parameters can be applied within variable practice conditions, and on which metrics can be considered useful for setting internal quality standards and derived benchmarks in the future. Although surgeons may be exposed to a variety of clinical practice needs, it appears from this experience that the proposed metrics as applied can serve as informative, reliable and consistent parameters to inform on quality-related aspects of surgical GC care and other conditions requiring gastrectomy.
Acknowledgements
None.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
Ethical Statement: This study was supported by IRB protocol No. 02200070019 of the University of Medicine and Dentistry of New Jersey, and by protocol No. STU 092010-072 of the University of Texas Southwestern Medical Center, with written informed consent obtained as required by the IRB.
References
- Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin 2017;67:7-30. [Crossref] [PubMed]
- Jemal A, Ward EM, Johnson CJ, et al. Annual Report to the Nation on the Status of Cancer, 1975-2014, Featuring Survival. J Natl Cancer Inst 2017;109. [Crossref] [PubMed]
- Guller U, Tarantino I, Cerny T, et al. Population-based SEER trend analysis of overall and cancer-specific survival in 5138 patients with gastrointestinal stromal tumor. BMC Cancer 30 2015;15:557.
- Stewart AK, Bland KI, McGinnis LS Jr, et al. Clinical highlights from the National Cancer Data Base, 2000. CA Cancer J Clin 2000;50:171-83. [Crossref] [PubMed]
- Strong VE, Song KY, Park CH, et al. Comparison of gastric cancer survival following R0 resection in the United States and Korea using an internationally validated nomogram. Ann Surg 2010;251:640-6. [Crossref] [PubMed]
- Selby LV, Vertosick EA, Sjoberg DD, et al. Morbidity after Total Gastrectomy: Analysis of 238 Patients. J Am Coll Surg 2015;220:863-71.e2. [Crossref] [PubMed]
- Randle RW, Swords DS, Levine EA, et al. Optimal extent of lymphadenectomy for gastric adenocarcinoma: A 7-institution study of the U.S. gastric cancer collaborative. J Surg Oncol 2016;113:750-5. [Crossref] [PubMed]
- Smith JK, McPhee JT, Hill JS, et al. National outcomes after gastric resection for neoplasm. Arch Surg 2007;142:387-93. [Crossref] [PubMed]
- Papenfuss WA, Kukar M, Oxenberg J, et al. Morbidity and mortality associated with gastrectomy for gastric cancer. Ann Surg Oncol 2014;21:3008-14. [Crossref] [PubMed]
- Birkmeyer JD, Sun Y, Goldfaden A, et al. Volume and process of care in high-risk cancer surgery. Cancer 2006;106:2476-81. [Crossref] [PubMed]
- Birkmeyer JD, Sun Y, Wong SL, et al. Hospital volume and late survival after cancer surgery. Ann Surg 2007;245:777-83. [Crossref] [PubMed]
- Kaye DR, Norton EC, Ellimoottil C, et al. Understanding the relationship between the Centers for Medicare and Medicaid Services' Hospital Compare star rating, surgical case volume, and short-term outcomes after major cancer surgery. Cancer 2017;123:4259-67. [Crossref] [PubMed]
- Henry LR, von Holzen UW, Minarich MJ, et al. Quality measurement affecting surgical practice: Utility versus utopia. Am J Surg 2018;215:357-66. [Crossref] [PubMed]
- Ajani JA, D'Amico TA, Almhanna K, et al. Gastric Cancer, Version 3.2016, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2016;14:1286-312. [Crossref] [PubMed]
- Mortensen K, Nilsson M, Slim K, et al. Consensus guidelines for enhanced recovery after gastrectomy: Enhanced Recovery After Surgery (ERAS(R)) Society recommendations. Br J Surg 2014;101:1209-29. [Crossref] [PubMed]
- Raigani S, Hardacre JM, Kim J, et al. Trends in the surgical treatment of gastric adenocarcinoma. Ann Surg Oncol 2014;21:569-74. [Crossref] [PubMed]
- Szucs Z, Thway K, Fisher C, et al. Molecular subtypes of gastrointestinal stromal tumors and their prognostic and therapeutic implications. Future Oncol 2017;13:93-107. [Crossref] [PubMed]
- Kodera Y, Schwarz RE, Nakao A. Extended lymph node dissection in gastric carcinoma: where do we stand after the Dutch and British randomized trials? J Am Coll Surg 2002;195:855-64. [Crossref] [PubMed]
- Schwarz RE. Spleen-preserving splenic hilar lymphadenectomy at the time of gastrectomy for cancer: technical feasibility and early results. J Surg Oncol 2002;79:73-6. [Crossref] [PubMed]
- Schwarz RE. Current status of management of malignant disease: current management of gastric cancer. J Gastrointest Surg 2015;19:782-8. [Crossref] [PubMed]
- Smith DD, Schwarz RR, Schwarz RE. Impact of total lymph node count on staging and survival after gastrectomy for gastric cancer: data from a large US-population database. J Clin Oncol 2005;23:7114-24. [Crossref] [PubMed]
- Schwarz RE, Smith DD. Clinical impact of lymphadenectomy extent in resectable gastric cancer of advanced stage. Ann Surg Oncol 2007;14:317-28. [Crossref] [PubMed]
- Naffouje SA, Salti GI. Extensive Lymph Node Dissection Improves Survival among American Patients with Gastric Adenocarcinoma Treated Surgically: Analysis of the National Cancer Database. J Gastric Cancer 2017;17:319-30. [Crossref] [PubMed]
- In H, Solsky I, Palis B, et al. Validation of the 8th Edition of the AJCC TNM Staging System for Gastric Cancer using the National Cancer Database. Ann Surg Oncol 2017;24:3683-91.
- Jin LX, Moses LE, Squires MH 3rd, et al. Factors Associated With Recurrence and Survival in Lymph Node-negative Gastric Adenocarcinoma: A 7-Institution Study of the US Gastric Cancer Collaborative. Ann Surg 2015;262:999-1005. [Crossref] [PubMed]
- Morgan JW, Ji L, Friedman G, et al. The role of the cancer center when using lymph node count as a quality measure for gastric cancer surgery. JAMA Surg 2015;150:37-43. [Crossref] [PubMed]
- Bond-Smith G, Belgaumkar AP, Davidson BR, et al. Enhanced recovery protocols for major upper gastrointestinal, liver and pancreatic surgery. Cochrane Database Syst Rev 2016;2. [PubMed]
- Lee MG, Chiu CC, Wang CC, et al. Trends and Outcomes of Surgical Treatment for Colorectal Cancer between 2004 and 2012- an Analysis using National Inpatient Database. Sci Rep 2017;7:2006. [Crossref] [PubMed]
- Schwarz RE, Karpeh MS, Brennan MF. Factors predicting hospitalization after operative treatment for gastric carcinoma in patients older than 70 years. J Am Coll Surg 1997;184:9-15. [PubMed]
- Squires MH 3rd, Kooby DA, Poultsides GA, et al. Effect of Perioperative Transfusion on Recurrence and Survival after Gastric Cancer Resection: A 7-Institution Analysis of 765 Patients from the US Gastric Cancer Collaborative. J Am Coll Surg 2015;221:767-77. [Crossref] [PubMed]
- Yang YS, Chen LQ, Yan XX, et al. Preservation versus non-preservation of the duodenal passage following total gastrectomy: a systematic review. J Gastrointest Surg 2013;17:877-86. [Crossref] [PubMed]
- Abdel-Misih SR, Wei L, Benson AB 3rd, et al. Neoadjuvant Therapy for Rectal Cancer Affects Lymph Node Yield and Status Without Clear Implications on Outcome: The Case for Eliminating a Metric and Using Preoperative Staging to Guide Therapy. J Natl Compr Canc Netw 2016;14:1528-34. [Crossref] [PubMed]
- Ding J, Sun B, Song P, et al. The application of enhanced recovery after surgery (ERAS)/fast-track surgery in gastrectomy for gastric cancer: a systematic review and meta-analysis. Oncotarget 2017;8:75699-711. [PubMed]
- Manso M, Schmelz J, Aloia T. ERAS-Anticipated outcomes and realistic goals. J Surg Oncol 2017;116:570-7. [Crossref] [PubMed]
- Mason MC, Chang GJ, Petersen LA, et al. National Quality Forum Colon Cancer Quality Metric Performance: How Are Hospitals Measuring Up? Ann Surg 2017;266:1013-20. [Crossref] [PubMed]
- Karanicolas PJ, Bickenbach K, Jayaraman S, et al. Measurement and interpretation of patient-reported outcomes in surgery: an opportunity for improvement. J Gastrointest Surg 2011;15:682-9. [Crossref] [PubMed]
- Straatman J, van der Wielen N, Joosten PJ, et al. Assessment of patient-reported outcome measures in the surgical treatment of patients with gastric cancer. Surg Endosc 2016;30:1920-9. [Crossref] [PubMed]
- Bilimoria KY, Bentrem DJ, Stewart AK, et al. Lymph node evaluation as a colon cancer quality measure: a national hospital report card. J Natl Cancer Inst 2008;100:1310-7. [Crossref] [PubMed]
- Cipriano A, Burfeind WR Jr. National Quality Forum Metrics for Thoracic Surgery. Thorac Surg Clin 2017;27:245-9. [Crossref] [PubMed]
Cite this article as: Schwarz RE. Clinical trends and effects on quality metrics for surgical gastroesophageal cancer care. Transl Gastroenterol Hepatol 2018;3:43.


