Role of inflammatory markers as hepatocellular cancer selection tool in the setting of liver transplantation
Introduction
Hepatocellular carcinoma (HCC) is the second leading cause of cancer-related mortality, accounting for over 600,000 deaths annually worldwide (1). Surgical treatment for HCC initially consisted of resection but soon grew to include liver transplantation (LT). Results of LT for HCC were initially disappointing with unsatisfactory outcomes due to poor candidate selection, likely related to a limited understanding of tumor biology (2,3). In 1996, Mazzaferro et al. introduced the Milan criteria to identify appropriate LT candidates with HCC based on the size and number of tumors (4). Soon after in 2002, the United Network for Organ Sharing (UNOS) adopted these criteria for patient selection for listing with exception points in the United States, resulting in massive changes in the proportion of patients with HCC being transplanted (5,6). The Milan criteria, however rely solely on imaging characteristics which are prone to error, with discordance in both size and number reported when compared to explant pathology (7-10). Notably, in Mazzaferro et al.’s landmark paper, 27% of patients who appeared within Milan criteria at the time of LT were found to have tumors outside of criteria on explant pathology (4). However, most importantly, imaging is unable to characterize patients who are at high risk of recurrence due to unfavorable tumor biology (9). While patients with moderate or poorly differentiated tumors have a higher risk of microvascular invasion (11) and recurrence after LT (12), those with lower tumor grades have a lower risk of recurrence even when outside the Milan criteria (13). Biopsies pose a risk of seeding the tumor (14), and obtaining one on every patient with HCC is unnecessary and inappropriate (15). Given the recurrence rate of HCC is as high as 15–20% in some series (16-18), assessing tumor biology and risk of recurrence noninvasively is essential.
Biomarkers may prove to be the missing link by providing the biological information that current imaging techniques lack. Biomarkers are defined as an objectively measured characteristic to indicate a biological process or response (19). They have the potential to assess the risk of recurrence in HCC without invasive testing, offering yet another tool in a physician’s armamentarium to identify the patients who would benefit from LT and those who would not. Currently, several biomarkers are utilized in clinical practice, but both the AASLD and an international consensus (15,20) describe biomarkers as an area of future study. Alpha fetoprotein (AFP) (21-24) and proteins induced by vitamin K absence II (PIVKA-II) (25-28) have been extensively studied and validated as predictors of HCC outcomes. However, there are patients who do not produce either biomarker and still have high risk of recurrence (29,30). Therefore, pursuing other biomarkers that assess the role of inflammation in malignancy is another method to identify patients at risk of recurrence. The link between inflammation and cancer was postulated in the year 1863 by Virchow, and inflammation has been linked with several gastrointestinal malignancies (31). In the last decade, there has been increasing interest in the use of inflammatory markers as tools to predict poor outcomes in patients with HCC. This review will focus of such inflammatory markers, the role they play in HCC, and how to potentially use these as selection tools for patients awaiting transplant.
Neutrophil lymphocyte ratio (NLR)
The NLR is a measure of the proportion of peripheral blood neutrophils to lymphocytes and has an important role in predicting outcomes in several malignancies. An elevated NLR can be a marker of both neutrophilia in response to inflammation or peripheral lymphopenia, both of which have been linked to poor outcomes in malignancy. NLR has been a biomarker associated with poor outcomes in various solid tumors, first noted in colorectal cancer. Walsh et al. noted that pre-operative NLR was associated with poor overall survival (OS) and recurrence-free survival (RFS) in patients with colorectal cancer (32). Halazun et al. were the first to link NLR with liver cancer when they analyzed patients undergoing resection of liver metastasis from colorectal cancer and noted that elevated NLR was associated with increased risk of recurrence and death (33). Further studies have confirmed NLR to be an accurate predictor of outcomes in a variety of other solid tumors (34) including esophageal cancer (35). The use of NLR quickly expanded to HCC and has proven effective as a biomarker to predict outcomes for a variety of therapies.
NLR has been extensively studied for in patients with HCC who undergo LT (36-41) (Table 1). Halazun et al. first demonstrated the use of NLR in predicting outcomes for HCC in 150 patients undergoing LT for HCC. Of the 13 patients with elevated NLR, 62% had recurrence and, as a cohort, had significantly worse OS and RFS. Of note, even patients within Milan criteria with an elevated NLR, compared to those with normal NLR, had a significantly worse survival (30% vs. 81%) (36). In the largest study in the MELD era from the United States examining post-LT outcomes for HCC, the MORAL score was evaluated for its ability to predict post-LT RFS. The MORAL score was constructed using Cox-regression analyses for factors impacting RFS post-transplant in patients with HCC at a single institution. Three scores were constructed, a pre-MORAL score consisting of only preoperatively available data, the post MORAL score consisting of pathological data and the combined MORAL score, which joined both pre- and post-scores. The pre-MORAL score, which was significantly more accurate in predicting RFS than the Milan criteria, consisted of pre-LT measures, namely preoperative NLR, maximum AFP >200 ng/mL, and largest tumor size. Compared to the Milan criteria the C-statistic fit the Pre-MORAL score was 0.82 compared to 0.63 for Milan. The post-MORAL included post-LT pathologic measures, namely tumor number, grade, size, and vascular invasion. NLR was the most predictive factor, along with grade 4 tumors, of poor RFS post-LT (43). Most importantly, the NLR based Pre-MORAL score identified patients with high risk of recurrence who had small tumors (within Milan criteria), showing that the inflammatory response to these tumors may be a key to understanding HCC tumor biology. In Asia, the NLR has been equally effective in predicting poor outcomes for living donor LT. Motomura et al. studied 158 patients who underwent living donor LT for HCC and noted that elevated NLR was associated with significantly worse RFS in all patients and that RFS was inversely related to NLR. Additionally, outcomes were worse for those within Milan criteria and especially in those who exceeded Milan criteria, with a RFS of 0% vs. 76% (38). Another study of 152 patients receiving living donor LT for HCC assessed for risk factors associated with poor outcomes in patients that already had a PIVKA-II level <300 mAU/mL and tumor size <5 cm. An elevated NLR was associated with a seven-fold increase in tumor recurrence and was more predictive than AFP in that cohort (39). While an increased NLR demonstrates clear predictive ability, the degree of elevation and trend are important. Harimoto et al. studied 167 patients who underwent living donor LT for HCC and noted that in patients who died post-LT, NLR was re-elevated, while those who survived had a gradual decrease in NLR. No patients in that study who had an elevated NLR survived to 3 years (42). Another large study of patients with LT for HCC noted that as NLR increased each log unit, the risk of HCC recurrence nearly doubled (41).

Full table
Not only has NLR been demonstrated to be predictive of survival in patients undergoing LT for HCC, it has been successful in evaluating patients undergoing tumor downstaging procedures such as radiofrequency ablation (RFA) (44-46) and transcatheter arterial chemoembolization (TACE) (47). In a study of 163 patients, most of which had viral hepatitis, with HCC treated with RFA, NLR post-RFA was strongly predictive of survival. However, pre-RFA NLR was not associated with outcomes in this cohort. Additionally, while elevated NLR was predictive of recurrence in hepatitis B patients, it was not predictive in recurrence in hepatitis C patients (46). Dan et al. analyzed the effect of NLR in 178 patients with small HCC treated with RFA. In this cohort and in agreement with Tajiri et al., post-RFA NLR was predictive of OS while pre-RFA NLR was not. Additionally, a decrease in post-RFA NLR was more accurate in identifying those with improved OS than pre-RFA or post-RFA absolute values (45). Another study of 158 patients who underwent RFA for HCC found that NLR was associated with worse OS but not tumor recurrence. Of the 140 patients who had follow up NLR values post-RFA, elevated NLR was associated again with OS and recurrence in this cohort (44). Lai et al. analyzed 181 patients with HCC on the transplant waiting list and evaluated the effect of NLR. Elevated NLR was associated with increased waitlist drop-off and worse OS. Approximately half of the patients with elevated NLR who dropped off the waitlist had progression of HCC. In this cohort, a subgroup analysis of 134 patients undergoing locoregional therapy demonstrated that elevated NLR was also associated with poor OS (48). A meta-analysis of the effect of NLR on patients with HCC identified a pooled hazard ratios (HR) for OS of 1.28 and 2.52 for those who underwent RFA and TACE, respectively (49).
In patients who are ineligible for LT with advanced and unresectable HCC, NLR can predict outcomes and response to various treatment modalities. McNally et al. evaluated the accuracy of NLR in predicting poor outcomes in 103 patients receiving TACE for unresectable HCC. Median survival for those with elevated pre-TACE NLR was 4 months compared to 15 months in those with normal NLR. If NLR remained elevated or rose further after TACE, outcomes were equally poor (50). Sukato et al. found similar results when analyzing 176 patients with unresectable HCC undergoing yttrium-90 radioembolization (51). In 145 patients receiving sorafenib for advanced HCC, low NLR was associated with improved survival (52). Other studies have confirmed worse OS associated with NLR in patients taking sorafenib (53,54). Terashima et al. found that in 266 patients undergoing hepatic arterial infusion chemotherapy, elevated NLR was associated with a significantly worse response rate (55). Similar findings were revealed by Tajiri et al. in 26 patients in which patients with elevated NLR were half as likely to respond to hepatic arterial infusion chemotherapy (56). Finally, elevated NLR in patients receiving selective internal radiation therapy (57) and those who underwent resection for tumors at least 10 cm in size (58) had significantly worse outcomes if NLR was elevated.
One of the mechanisms proposed for the importance of neutrophilia in malignancy is neutrophil secretion of vascular endothelial growth factor (VEGF), a known promoter of angiogenesis (59-62) that is important in several cancer types including non-small cell lung cancer (63), colorectal cancer (64), and HCC (65,66). A study by Miura et al. noted that VEGF mRNA was upregulated in 10 of the 12 cases with abundant tumor vascularity (65). VEGF can be potentiated by multiple cytokines released by neutrophils including tumor necrosis alpha and other interleukins (67). In fact, elevated intratumoral neutrophils in comparison to CD8+ T cells in patients with HCC was a strong predictor of poor outcomes, even in patients with normal AFP (68). An elevated NLR in the peritumoral tissue was also correlated with poor outcomes in patients with HCC (69) Furthermore, peripheral NLR was noted by Motomura et al. to be associated with elevated peritumoral interleukin-17 (IL-17) (38). IL-17 itself has been associated with worse OS and increased risk of recurrence as it has been shown to upregulate tumor angiogenesis (70-72). On the other hand, overall lymphopenia has been associated with poor outcomes after LT as well (73). Additionally, elevated AFP, microvascular invasion, and presence of multifocal tumors and extrahepatic metastasis, all factors associated with poor outcomes in HCC patients, are significantly associated with NLR (49,72,74).
While NLR is clearly predictive of outcomes in patients undergoing LT or receiving treatment for downstaging HCC, its likely destiny is as an adjunct to other well-validated criteria in determining LT candidacy. In multiple studies and most notably in the MORAL score, NLR is a potent predictor post-LT outcome. Based on these findings, other studies that have added NLR as an adjunct to known risk factors for recurrence such as the Milan criteria (75) and tumor size and number (39) and have had improved accuracy in predicting poor outcomes and hopefully improving future patient selection. NLR is routine, safe, and easy to obtain compared to other data that is equally informative, such as pathological tumor grading. Conversely, it can also be affected by other inflammatory conditions such as acute infection or hematologic disorders. Ideally, NLR can help classify patients as higher risk for recurrence of HCC after LT and lead physicians to consider obtaining further information, especially pathologic data by way of biopsy, to help further prognosticate. Future studies that evaluate more extensively used biomarkers, such as AFP and PIVKA-II, could be combined with NLR to create a model with even more accurate pre-LT evaluation.
C-reactive protein (CRP)
CRP is an acute-phase reactant that has gained significant clinical value as a marker of acute and chronic inflammation. It is one of several acute-phase proteins synthesized primarily by hepatocytes under the regulation and in response to interleukin-1 (IL-1) and interleukin-6 (IL-6) (76-78). CRP is a sensitive inflammatory marker, increasing by up to a hundred-fold in response to inflammation (76), although the rise in levels can be very non-specific, responding to different states such as infection, injury or malignancy.
There has been growing interest in the role of CRP in the prognostication of malignancy, including its role in predicting HCC outcomes (78). The cellular biology involved in regulating and sustaining tumor growth has in part included a focus on inflammation. Necrotic cell death seen in the of background of malignancy leads to the release of the mediators of inflammation, including IL-1, into the cells’ surrounding environment, promoting angiogenesis and thus tumor cell growth (79). Therefore, on a molecular level, there appears to be a link between the regulatory signals of CRP production, specifically hepatocyte necrosis leading to release of IL-1 promoting IL-6 production (80), and cancer cell growth and invasion.
Within the oncologic community, clinically meaningful outcomes in various malignancies have been linked to CRP levels (81). In a large prospective, nested case-control study of a 22,887-patient cohort, Erlinger et al. demonstrated the value of baseline CRP levels in predicting the incidence of colorectal cancer. Individuals with baseline CRP levels in the highest quartile had over twice the risk of colon cancer compared to those with lower CRP levels (82).
The value of CRP in HCC prognostication has been duplicated and confirmed in various stages of the disease (78,83). It has not only been shown to be predictive of tumor characteristics histologically, but also a useful tool in predicting response to various treatment options and long-term survival afterwards (83,84).
HCC histologic evaluation provides vital information helpful in predicting risk of progression and relapse after treatment, including after LT. Careful examination of the number, size and staging of HCC on explanted livers after transplantation or after tumor resection is common practice among many large medical centers to help guide tumor surveillance. Given the strong link between inflammation and the cellular mechanisms of tumor progression in HCC, several investigators have examined the link between CRP and tumor histology and disease stage. Zheng et al. performed a meta-analysis of ten studies including 1,885 patients in total examining the role of CRP levels in predicting the prognosis of patients with HCC. The results overwhelmingly supported the value of CRP levels given its correlation with pathology. CRP expression ≥10 mg/L was associated with more aggressive tumor biology, including larger tumors (OR 3.41), vascular invasion (OR 3.05), >1 tumor (OR 2.36) and higher TMN staging (OR 3.23) (78). In the same meta-analysis, pooled analysis demonstrated that poor OS and RFS in HCC was significantly associated with CRP ≥10 mg/L, with a HR of 2.15 and 2.66 respectively. Similarly, Kinoshita et al., in two separate studies investigating scoring systems driven by CRP (83,84), again demonstrated the strong correlation between extent and stage of disease and the Glasgow prognostic score (GPS) and CRP/albumin ratio. One hundred and fifty individuals with newly diagnosed HCC at various stages were prospectively followed and divided based on their GPS and modified GPS. A higher GPS significantly correlated with tumor number, vascular invasion, maximal tumor diameter and frequency of extra hepatic metastasis (83). In the second study, which retrospectively investigated incorporating albumin with CRP using the CRP/albumin ratio to improve the prognostic value of both markers, the CRP/albumin ratio, based on a cutoff of <0.037, was associated with larger tumor size and vascular invasion. Additionally, it was associated with extrahepatic metastasis, a higher Cancer of the Liver Italian Program (CLIP) score and a higher Barcelona Clinic Liver Cancer (BCLC) stage (84). As expected, OS was significantly lower at 1, 3 and 5 years in individuals with CRP/albumin ratio ≥0.037. Portal vein invasion identified after resection has also correlated with higher CRP levels pre-operatively when studied in 141 patients undergoing curative resection for HCC. The 1-, 3- and 5-year survival rates in the CRP-positive group was much lower when compared to the CRP-negative group, with 5-year survival differing by as much as 53.4%. Recurrence free survival shared a similar trend, with CRP-positive patients experiencing a 75.3% recurrence within 1 year, even when curative resection was determined by negative surgical margins (81). It therefore appears that markers of active inflammation, and more specifically higher CRP levels, consistently correlate with burden of HCC and therefore, poorer long-term outcomes.
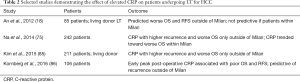
The demonstrated predictive value of CRP levels in patients with HCC in terms of histologic extent and aggressiveness of disease has generated increased interest and active investigation into its role in prognostication post-LT, possibly providing a pre-LT prognostic tool to allow clinicians to better risk stratify patients. Data on both pre- and post-LT CRP values in predicting outcomes in LT for HCC exists (Table 2). An et al. persuasively demonstrated the usefulness of pre-liver transplant serum CRP levels in predicting outcomes after transplantation for HCC (18). Eighty-five patients who underwent LT for HCC (the vast majority were living donor LT) with available pre-transplant CRP levels were followed for a median period of 28.3 months. Forty percent of the patients had HCC beyond Milan criteria at the time of transplant. A high CRP (≥1 mg/dL) was a significant independent risk factor for OS (HR 2.68) and tumor recurrence (HR 4.64). Interestingly, the correlation between CRP with both OS and RFS after LT was limited to patients beyond Milan criteria, a higher risk population that would benefit from more detailed risk stratification, potentially allowing more refined access to safe transplant. A similar trend was demonstrated by Kim et al. in 211 liver transplant recipients for HCC (again the majority living donor LT). An elevated CRP level prior to LT was associated with higher tumor recurrence and poor OS, an association that was only seen in patients beyond Milan criteria (HR 4.67 for tumor reoccurrence and HR 4.32 for OS) (85). Similar to prior studies, the high CRP group had significantly higher Child-Pugh class, MELD at transplant, and maximal tumor size. Work from another group in South Korea with the aim to establish a new pre-LT scoring model utilizing both NLR and CRP, further reinforced the value of incorporating this biomarker. Two hundred and forty-two individuals with HCC, of which only 59% were within Milan criteria, underwent living donor LT. On univariate analysis only, pre-transplant CRP was significantly associated with HCC recurrence and OS in the entire study population. As observed in other studies, for patients beyond Milan criteria, NLR and CRP were both associated with OS and RFS, though CRP failed to show this trend in multivariate analysis. In contrast to previously described studies, a higher CRP did show a trend towards decreased OS in patients within Milan criteria (P=0.054) (75). Similar to pre-LT CRP, measurement of post-LT CRP seems to have prognostic value in predicting outcomes in HCC. Kornberg et al. demonstrated retrospectively in 106 liver transplant recipients with HCC that peak early post-operative CRP was a significant predictor of poor OS. With a CRP cut off of 3.5 mg/dL representing a high CRP-population, the observed HCC recurrence after LT in this group was 48.7%, significantly higher than the 7.8% recurrence observed in patients with peak CRP level <3.5 mg/dL. The 1-, 3-, and 5-year OS and RFS were significantly lower in the high CRP-group, and a higher early post-LT CRP was predictive of increased tumor recurrence in patients outside of Milan criteria (86). Hence, CRP has consistently proven to be a valuable inflammatory biomarker, when measured both before and shortly after LT, in predicting clinically meaningful outcomes in liver transplant recipients with HCC.
Full table
Locoregional therapy is often utilized for patients with HCC both within and outside of Milan criteria either to control disease while awaiting transplantation or for downstaging purposes. Aside from AFP, biomarkers are not commonly used in practice to predict response to locoregional treatment. However, there has been some interest in investigating the utility of CRP in this domain. Li et al. explored the prognostic value of CRP in patients with HCC after TACE. One hundred and seventeen patients with intermediate or advanced-stage HCC who underwent conventional TACE were followed for 3 years after treatment to evaluate OS. A lower CRP level was significantly associated with higher OS at 1, 2, and 3 years, though at 3 years the OS was still low at 20.83% (87). Similarly, a French study of 157 patients with cirrhosis and HCC undergoing first time TACE/TAE confirmed that a higher CRP predicted poorer OS after treatment, with more individuals exceeding the “up-to-7 criteria” in the high CRP group (88). Given the utility of CRP in HCC prognostication, Pinato et al. utilized the inflammation-based index (IBI), a score derived from CRP and albumin levels, to explore its prognostic ability in post-TACE survival. A higher IBI at baseline demonstrated lower median survival at follow up and a normalization of IBI after TACE remained an independent predictor of better OS (89). Similar data has also been established in RFA for HCC. A study enrolling 387 patients with 3 or less HCC nodules all under 3 cm in size for RFA demonstrated that better OS and decreased tumor recurrence was tied to a lower hsCRP level (adjusted HR 1.32 for tumor recurrence and adjusted HR 1.59 for survival) (90).
For patients with more advanced HCC, where both transplantation and locoregional therapy are not options, CRP levels may be helpful in differentiating individuals in terms of potential length of survival. 81 patients with advanced HCC in Japan receiving sorafenib were divided based on GPS, an inflammatory based index that incorporates CRP levels. A multivariate analysis revealed that patients with a higher score had a significantly shorter OS (HR 5.483), suggesting potential prognostic value of CRP levels in this patient population (91). Nakanishi et al. echoed these results when investigating potential prognostic markers in 165 patients with advanced HCC receiving sorafenib. The group characterized by a higher CRP level had significantly poorer OS, with a median survival time differing by as much as 10 months between the two groups of patients (92).
Platelet lymphocyte ratio (PLR)
PLR has only recently been used as an inflammatory biomarker for assessing HCC outcomes post-LT since 2012 by Pinato et al. (Table 3). The authors studied a training set of 112 patients with mostly unresectable HCC and identified that elevated PLR was associated with over twofold decrease in OS. PLR was then re-evaluated in a validation cohort of 466 patients with HCC with lower tumor burden and found to be comparable in predicting OS to NLR (93). Another cohort of 343 patients undergoing LT for HCC were analyzed for the impact of pre-LT PLR on outcomes. High PLR was associated with a reduced RFS and accurately identified patients outside of Milan and UCSF criteria. Of note, elevated PLR was associated with more advanced pathologic data including larger tumor size, multiple tumors, and microvascular and macrovascular tumor invasion (94). Finally, in the study by Lai et al. above, in the same cohort that noted that NLR was associated with increased LT waitlist drop-off, PLR was shown to be more effective in predicting RFS and tumor recurrence post-LT than NLR (48). However, it is worth noting that this was the rare study that showed PLR to be superior to NLR.

Full table
Although there is limited data on the accuracy of PLR prior to downstaging procedures such as TACE, the studies that were performed consistently showed that elevated PLR is predictive of poor outcomes. Fan et al. examined the impact of PLR and NLR on a cohort of 132 patients with recurrent HCC receiving TACE. Both elevated NLR and PLR were associated with worse OS but PLR was a more accurate predictor of 1-year survival (95). Another study evaluating the predictive ability of PLR in post-TACE outcomes analyzed 122 patients with HBV-related HCC. Patients with elevated PLR had significantly worse OS, and elevated PLR was noted to be even more impactful than NLR, AFP, and tumor or nodal status (96). Xue et al. examined outcomes in 291 patients receiving multiple TACE procedures for huge HCC tumors (diameter >10 cm). Elevated PLR was associated with worse OS in this cohort (97).
The most extensive data exists linking PLR and post-resection outcomes. Peng et al. evaluated 219 patients with HBV-related HCC who underwent curative hepatic resection and identified patients with a significant increase in PLR post-resection. The cohort with a significant increase in PLR had had worse OS and RFS (98). Kaida et al. studied 271 patients who underwent curative hepatic resection, had recurrence, and differentiated patients who had recurrence within and outside of Milan criteria. PLR was found to be predictive of recurrence outside of Milan criteria and was associated with poorly differentiated tumors that were larger and had a higher PIVKA-II (99). In huge HCC tumors undergoing resection, Goh et al. noted that an elevated PLR was associated with a decreased OS and RFS. In summary, Zhao et al. performed a meta-analysis of ten studies, with a wide variety of treatments ranging from surgical resection to TACE but not including LT, and analyzed the effect of PLR on outcomes in HCC. The authors found that elevated PLR was associated with worse OS but not RFS (100).
Similar to NLR, the PLR is a simple laboratory test that measures the proportion of platelets to lymphocytes and can be a marker of either thrombocytopenia and/or relative lymphopenia. Long known as a marker of inflammation, platelets exacerbate the inflammatory response by releasing proteins such as platelet-derived growth factor and transforming growth factor α and β (31). Platelets themselves have been implicated in stimulating tumor growth and angiogenesis by releasing several tumor-promoting enzymes, most notably VEGF. Additionally, platelets are instrumental in blocking tumor cell removal by the immune system and can help establish metastatic lesions (101). An elevated PLR has been associated with larger tumor size, PIVKA-II, portal vein thrombosis, aggressive tumor behavior, advanced HCC tumor stage, and distant metastasis (94,99,102).
Despite only being studied for HCC since 2012, PLR has been useful in identifying patients at higher risk of worse outcomes. PLR, like NLR, is easily obtainable, but has not been studied as extensively. Elevation of the PLR, given its dependence on thrombocytosis, correlates with a significant increase in the inflammatory state. However, at this time, PLR is a helpful but still somewhat limited adjunct. Given the paucity of evidence supporting its importance, the widespread use of this biomarker cannot be fully recommended.
Conclusions
HCC is a leading cause of cancer-related death across the world. Among several options for treatment, LT stands out as it is potentially curative and has become increasingly more common and successful. Major strides have been made in survival and recurrence since the widespread use of the Milan criteria, but recurrence rates still remain high. Noninvasive measurements of inflammatory biomarkers offer significant potential as adjuncts to the more established Milan criteria. Influential scoring systems for HCC recurrence, such as the MORAL score (42), have already incorporated NLR as a key component in its calculation to predict HCC recurrence after LT. Our review has demonstrated that NLR has been proven effective in not only predicting post-LT recurrence of HCC but also in locoregional therapies that typically precede LT. CRP is another commonly obtained laboratory test that can help stratify patients for risk of recurrence of HCC, but at this point, appears to be most helpful when patients are outside of the Milan criteria. On the other hand, PLR still requires further investigation into its efficacy as a biomarker for predicitng outcomes in patients with HCC. In summary, there is a significant role for inflammatory biomarkers to play and their incorporation into scoring systems that account for tumor size and number will only improve our ability to predict outcomes after HCC.
Acknowledgements
The authors would like to acknowledge the assistance of the department of surgery and the division of gastroenterology and hepatology for their support.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Jemal A, Siegel R, Xu J, et al. Cancer Statistics, 2010. CA Cancer J Clin 2010;60:277-300. [Crossref] [PubMed]
- Iwatsuki S, Gordon RD, Shaw BW Jr, et al. Role of liver transplantation in cancer therapy. Ann Surg 1985;202:401-7. [Crossref] [PubMed]
- O’Grady JG, Polson RJ, Rolles K, et al. Liver transplantation for malignant disease. Results in 93 consecutive patients. Ann Surg 1988;207:373-9. [Crossref] [PubMed]
- Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med 1996;334:693-9. [Crossref] [PubMed]
- UNOS/OPTN policy. Available online: https://optn.transplant.hrsa.gov/media/1200/optn_policies.pdf
- Ha J, Yan M, Aguilar M, et al. Race/ethnicity-specific disparities in cancer incidence, burden of disease, and overall survival among patients with hepatocellular carcinoma in the United States. Cancer 2016;122:2512-23. [Crossref] [PubMed]
- Sotiropoulos GC, Malag M, Molmenti E, et al. Liver Transplantation for Hepatocellular Carcinoma in Cirrhosis: Is Clinical Tumor Classification before Transplantation Realistic? Transplantation 2005;79:483-7. [Crossref] [PubMed]
- Shah SA, Tan JC, McGilvray ID, et al. Accuracy of staging as a predictor for recurrence after liver transplantation for hepatocellular carcinoma. Transplantation 2006;81:1633-9. [Crossref] [PubMed]
- Schwartz ME, D’Amico F, Vitale A, et al. Liver transplantation for hepatocellular carcinoma: Are the Milan criteria still valid? Eur J Surg Oncol 2008;34:256-62. [Crossref] [PubMed]
- Costentin CE, Amaddeo G, Decaens T, et al. Prediction of hepatocellular carcinoma recurrence after liver transplantation: Comparison of four explant-based prognostic models. Liver Int 2017;37:717-26. [Crossref] [PubMed]
- Esnaola NF, Lauwers GY, Mirza NQ, et al. Predictors of microvascular invasion in patients with hepatocellular carcinoma who are candidates for orthotopic liver transplantation. J Gastrointest Surg 2002;6:224-32. [Crossref] [PubMed]
- Jonas S, Bechstein WO, Steinmüller T, et al. Vascular invasion and histopathologic grading determine outcome after liver transplantation for hepatocellular carcinoma in cirrhosis. Hepatology 2001;33:1080-6. [Crossref] [PubMed]
- Cillo U, Vitale A, Bassanello M, et al. Liver transplantation for the treatment of moderately or well-differentiated hepatocellular carcinoma. Ann Surg 2004;239:150-9. [Crossref] [PubMed]
- Silva MA, Hegab B, Hyde C, et al. Needle track seeding following biopsy of liver lesions in the diagnosis of hepatocellular cancer: a systematic review and meta-analysis. Gut 2008;57:1592-6. [Crossref] [PubMed]
- Bruix J, Sherman M. AASLD Practice Guideline, Management of Hepatocellular Carcinoma: An Update. Hepatology 2010;42:1-35.
- Bodzin AS, Lunsford KE, Markovic D, et al. Predicting Mortality in Patients Developing Recurrent Hepatocellular Carcinoma After Liver Transplantation: Impact of Treatment Modality and Recurrence Characteristics. Ann Surg 2017;266:118-25. [Crossref] [PubMed]
- Park MS, Lee KW, Suh SW, et al. Living-Donor Liver Transplantation Associated With Higher Incidence of Hepatocellular Carcinoma Recurrence Than Deceased-Donor Liver Transplantation. Transplantation 2014;97:71-7. [Crossref] [PubMed]
- An HJ, Jang JW, Bae SH, et al. Serum C-reactive protein is a useful biomarker for predicting outcomes after liver transplantation in patients with hepatocellular carcinoma. Liver Transpl 2012;18:1406-14. [Crossref] [PubMed]
- Kessler LG, Barnhart HX, Buckler AJ, et al. The emerging science of quantitative imaging biomarkers terminology and definitions for scientific studies and regulatory submissions. Stat Methods Med Res 2015;24:9-26. [Crossref] [PubMed]
- Clavien PA, Lesurtel M, Bossuyt PM, et al. Recommendations for liver transplantation for hepatocellular carcinoma: an international consensus conference report. Lancet Oncol 2012;13:e11-22. [Crossref] [PubMed]
- Notarpaolo A, Layese R, Magistri P, et al. Validation of the AFP model as a predictor of HCC recurrence in patients with viral hepatitis-related cirrhosis who had received a liver transplant for HCC. J Hepatol 2017;66:552-9. [Crossref] [PubMed]
- Berry K, Ioannou GN. Serum alpha-fetoprotein level independently predicts posttransplant survival in patients with hepatocellular carcinoma. Liver Transpl 2013;19:634-45. [Crossref] [PubMed]
- Dumitra TC, Dumitra S, Metrakos PP, et al. Pretransplantation α-fetoprotein slope and milan criteria: strong predictors of hepatocellular carcinoma recurrence after transplantation. Transplantation 2013;95:228-33. [Crossref] [PubMed]
- Hakeem AR, Young RS, Marangoni G, et al. Systematic review: the prognostic role of alpha-fetoprotein following liver transplantation for hepatocellular carcinoma. Aliment Pharmacol Ther 2012;35:987-99. [PubMed]
- Poté N, Cauchy F, Albuquerque M, et al. Performance of PIVKA-II for early hepatocellular carcinoma diagnosis and prediction of microvascular invasion. J Hepatol 2015;62:848-54. [Crossref] [PubMed]
- Fujiki M, Takada Y, Ogura Y, et al. Significance of des-gamma-carboxy prothrombin in selection criteria for living donor liver transplantation for hepatocellular carcinoma. Am J Transplant 2009;9:2362-71. [Crossref] [PubMed]
- Shirabe K, Itoh S, Yoshizumi T, et al. The predictors of microvascular invasion in candidates for liver transplantation with hepatocellular carcinoma - With special reference to the serum levels of des-gamma-carboxy prothrombin. J Surg Oncol 2007;95:235-40. [Crossref] [PubMed]
- Marrero JA, Su GL, Wei W, et al. Des-gamma carboxyprothrombin can differentiate hepatocellular carcinoma from nonmalignant chronic liver disease in American patients. Hepatology 2003;37:1114-21. [Crossref] [PubMed]
- Tsai SL, Huang GT, Yang PM, et al. Plasma des-gamma-carboxyprothrombin in the early stage of hepatocellular carcinoma. Hepatology 1990;11:481-8. [Crossref] [PubMed]
- Taketa K. α‐fetoprotein: Reevaluation in hepatology. Hepatology 1990;12:1420-32. [Crossref] [PubMed]
- Coussens LM, Werb Z. Inflammation and cancer. Nature 2002;420:860-7. [Crossref] [PubMed]
- Walsh SR, Cook EJ, Goulder F, et al. Neutrophil-lymphocyte ratio as a prognostic factor in colorectal cancer. J Surg Oncol 2005;91:181-4. [Crossref] [PubMed]
- Halazun KJ, Aldoori A, Malik HZ, et al. Elevated preoperative neutrophil to lymphocyte ratio predicts survival following hepatic resection for colorectal liver metastases. Eur J Surg Oncol 2008;34:55-60. [Crossref] [PubMed]
- Templeton AJ, McNamara MG, Šeruga B, et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: A systematic review and meta-analysis. J Natl Cancer Inst 2014;106:dju124. [Crossref] [PubMed]
- Sharaiha RZ, Halazun KJ, Mirza F, et al. Elevated Preoperative Neutrophil:Lymphocyte Ratio as a Predictor of Postoperative Disease Recurrence in Esophageal Cancer. Ann Surg Oncol 2011;18:3362-9. [Crossref] [PubMed]
- Halazun KJ, Hardy MA, Rana AA, et al. Negative Impact of Neutrophil-Lymphocyte Ratio on Outcome After Liver Transplantation for Hepatocellular Carcinoma. Ann Surg 2009;250:141-51. [Crossref] [PubMed]
- Bertuzzo VR, Cescon M, Ravaioli M, et al. Analysis of factors affecting recurrence of hepatocellular carcinoma after liver transplantation with a special focus on inflammation markers. Transplantation 2011;91:1279-85. [Crossref] [PubMed]
- Motomura T, Shirabe K, Mano Y, et al. Neutrophil-lymphocyte ratio reflects hepatocellular carcinoma recurrence after liver transplantation via inflammatory microenvironment. J Hepatol 2013;58:58-64. [Crossref] [PubMed]
- Yoshizumi T, Ikegami T, Yoshiya S, et al. Impact of tumor size, number of tumors and neutrophil-to-lymphocyte ratio in liver transplantation for recurrent hepatocellular carcinoma. Hepatol Res 2013;43:709-16. [Crossref] [PubMed]
- Limaye AR, Clark V, Soldevila-Pico C, et al. Neutrophil-lymphocyte ratio predicts overall and recurrence-free survival after liver transplantation for hepatocellular carcinoma. Hepatol Res 2013;43:757-64. [Crossref] [PubMed]
- Agopian VG, Harlander-Locke M, Zarrinpar A, et al. A novel prognostic nomogram accurately predicts hepatocellular carcinoma recurrence after liver transplantation: Analysis of 865 consecutive liver transplant recipients. J Am Coll Surg 2015;220:416-27. [Crossref] [PubMed]
- Harimoto N, Shirabe K, Nakagawara H, et al. Prognostic factors affecting survival at recurrence of hepatocellular carcinoma after living-donor liver transplantation: with special reference to neutrophil/lymphocyte ratio. Transplantation 2013;96:1008-12. [Crossref] [PubMed]
- Halazun KJ, Najjar M, Abdelmessih RM, et al. Recurrence After Liver Transplantation for Hepatocellular Carcinoma: A New MORAL to the Story. Ann Surg 2017;265:557-64. [Crossref] [PubMed]
- Chen TM, Lin CC, Huang PT, et al. Neutrophil-to-lymphocyte ratio associated with mortality in early hepatocellular carcinoma patients after radiofrequency ablation. J Gastroenterol Hepatol 2012;27:553-61. [Crossref] [PubMed]
- Dan J, Zhang Y, Peng Z, et al. Postoperative neutrophil-to-lymphocyte ratio change predicts survival of patients with small hepatocellular carcinoma undergoing radiofrequency ablation. PLoS One 2013;8:e58184. [Crossref] [PubMed]
- Tajiri K, Baba H, Kawai K, et al. Neutrophil-to-lymphocyte ratio predicts recurrence after radiofrequency ablation in hepatitis B virus infection. J Gastroenterol Hepatol 2016;31:1291-9. [Crossref] [PubMed]
- Qi X, Li J, Deng H, et al. Neutrophil-to-lymphocyte ratio for the prognostic assessment of hepatocellular carcinoma: A systematic review and meta-analysis of observational studies. Oncotarget 2016;7:45283-301. [Crossref] [PubMed]
- Lai Q, Castro Santa E, Rico Juri JM, et al. Neutrophil and platelet-to-lymphocyte ratio as new predictors of dropout and recurrence after liver transplantation for hepatocellular cancer. Transpl Int 2014;27:32-41. [Crossref] [PubMed]
- Xiao WK, Chen D, Li SQ, et al. Prognostic significance of neutrophil-lymphocyte ratio in hepatocellular carcinoma: a meta-analysis. BMC Cancer 2014;14:117. [Crossref] [PubMed]
- McNally ME, Martinez A, Khabiri H, et al. Inflammatory markers are associated with outcome in patients with unresectable hepatocellular carcinoma undergoing transarterial chemoembolization. Ann Surg Oncol 2013;20:923-8. [Crossref] [PubMed]
- Sukato DC, Tohme S, Chalhoub D, et al. The Prognostic Role of Neutrophil-to-Lymphocyte Ratio in Patients with Unresectable Hepatocellular Carcinoma Treated with Radioembolization. J Vasc Interv Radiol 2015;26:816-24.e1. [Crossref] [PubMed]
- Bruixola G, Niño OM, Diaz-Beveridge R, et al. Baseline neutrophil-to-lymphocyte ratio (NLR) and early toxicity as prognostic factors in advanced hepatocellular carcinoma patients treated with sorafenib. J Clin Oncol 2015;33:e15159.
- Luè A, Bustamante FJ, Iñarrairaegui M, et al. Neutrophil-to-lymphocyte ratio is a predictor of one-year survival in patients with hepatocellular carcinoma receiving sorafenib. J Hepatol 2014;60:S401. [Crossref]
- Afshar A, Clarke H, Jackson-Wilding A, et al. Neutrophil lymphocyte ratio (NLR) at diagnosis is a predictor for survival in patients receiving sorafenib for advanced hepatocellular carcinoma (HCC): A large UK cohort. J Hepatol 2015;62:S442. [Crossref]
- Terashima T, Yamashita T, Iida N, et al. Blood neutrophil to lymphocyte ratio as a predictor in patients with advanced hepatocellular carcinoma treated with hepatic arterial infusion chemotherapy. Hepatol Res 2014;45:949-59. [Crossref] [PubMed]
- Tajiri K, Kawai K, Minemura M, et al. Neutrophil/lymphocyte ratio as a prognostic indicator of hepatic arterial infusion chemotherapy with arterial cisplatin plus continuous 5-fluorouracil. Hepatol Res 2015;45:755-63. [Crossref] [PubMed]
- D’Emic N, Engelman A, Molitoris J, et al. Prognostic significance of neutrophil-lymphocyte ratio and platelet-lymphocyte ratio in patients treated with selective internal radiation therapy. J Gastrointest Oncol 2016;7:269-77. [PubMed]
- Goh BKP, Kam JH, Lee SY, et al. Significance of neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio and prognostic nutrition index as preoperative predictors of early mortality after liver resection for huge (≥10 cm) hepatocellular carcinoma. J Surg Oncol 2016;113:621-7. [Crossref] [PubMed]
- Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med 2003;9:669-76. [Crossref] [PubMed]
- Carmeliet P, During A. Mechanisms of angiogenesis and arteriogenesis. Nat Med 2000;6:389-95. [Crossref] [PubMed]
- Kim KJ, Li B, Winer J, et al. Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumour growth in vivo. Nature 1993;362:841-4. [Crossref] [PubMed]
- Yancopoulos GD, Davis S, Gale NW, et al. Vascular-specific growth factors and blood vessel formation. Nature 2000;407:242-8. [Crossref] [PubMed]
- Sandler A, Gray R, Perry MC, et al. Paclitaxel–Carboplatin Alone or with Bevacizumab for Non–Small-Cell Lung Cancer. N Engl J Med 2006;355:2542-50. [Crossref] [PubMed]
- Warren RS, Yuan H, Matli MR, et al. Regulation by vascular endothelial growth factor of human colon cancer tumorigenesis in a mouse model of experimental liver metastasis. J Clin Invest 1995;95:1789-97. [Crossref] [PubMed]
- Miura H, Miyazaki T, Kuroda M, et al. Increased expression of vascular endothelial growth factor in human hepatocellular carcinoma. J Hepatol 1997;27:854-61. [Crossref] [PubMed]
- Yamaguchi R, Yano H, Iemura A, et al. Expression of vascular endothelial growth factor in human hepatocellular carcinoma. Hepatology 1998;28:68-77. [Crossref] [PubMed]
- Neufeld G, Cohen T, Gengrinovitch S, et al. Vascular endothelial growth factor (VEGF) and its receptors. FASEB J 1999;13:9-22. [PubMed]
- Li YW, Qiu SJ, Fan J, et al. Intratumoral neutrophils: A poor prognostic factor for hepatocellular carcinoma following resection. J Hepatol 2011;54:497-505. [Crossref] [PubMed]
- He G, Zhang H, Zhou J, et al. Peritumoural neutrophils negatively regulate adaptive immunity via the PD-L1/PD-1 signalling pathway in hepatocellular carcinoma. J Exp Clin Cancer Res 2015;34:141. [Crossref] [PubMed]
- Pollard JW. Opinion: Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer 2004;4:71-8. [Crossref] [PubMed]
- Liao R, Sun J, Wu H, et al. High expression of IL-17 and IL-17RE associate with poor prognosis of hepatocellular carcinoma. J Exp Clin Cancer Res 2013;32:3. [Crossref] [PubMed]
- Huang GQ, Zhu GQ, Liu YL, et al. Stratified neutrophil-to-lymphocyte ratio accurately predict mortality risk in hepatocellular carcinoma patients following curative liver resection. Oncotarget 2016;7:5429-39. [Crossref] [PubMed]
- Nagai S, Abouljoud MS, Kazimi M, et al. Peritransplant lymphopenia is a novel prognostic factor in recurrence of hepatocellular carcinoma after liver transplantation. Transplantation 2014;97:694-701. [PubMed]
- Xue TC, Zhang L, Xie XY, et al. Prognostic significance of the neutrophil-to-lymphocyte ratio in primary liver cancer: a meta-analysis. PLoS One 2014;9:e96072. [Crossref] [PubMed]
- Na GH, Kim DG, Han JH, et al. Inflammatory markers as selection criteria of hepatocellular carcinoma in living-donor liver transplantation. World J Gastroenterol 2014;20:6594-601. [Crossref] [PubMed]
- Dufour JF. C-reactive protein, a prognostic marker in hepatocellular carcinoma. Hepatology 2013;57:2103-5. [Crossref] [PubMed]
- Castell JV, Gómez-lechón MJ, David M, et al. Acute-phase response of human hepatocytes: Regulation of acute‐phase protein synthesis by interleukin-6. Hepatology 1990;12:1179-86. [Crossref] [PubMed]
- Zheng Z, Zhou L, Gao S, et al. Prognostic role of C-reactive protein in hepatocellular carcinoma: A systematic review and meta-analysis. Int J Med Sci 2013;10:653-64. [Crossref] [PubMed]
- Hanahan D, Weinberg RA. Hallmarks of cancer: The next generation. Cell 2011;144:646-74. [Crossref] [PubMed]
- Sakurai T, He G, Matsuzawa A, et al. Hepatocyte Necrosis Induced by Oxidative Stress and IL-1α Release Mediate Carcinogen-Induced Compensatory Proliferation and Liver Tumorigenesis. Cancer Cell 2008;14:156-65. [Crossref] [PubMed]
- Hashimoto K, Ikeda Y, Korenaga D, et al. The impact of preoperative serum C-reactive protein on the prognosis of patients with hepatocellular carcinoma. Cancer 2005;103:1856-64. [Crossref] [PubMed]
- Erlinger TP, Platz EA, Rifai N, et al. C-reactive protein and the risk of incident colorectal cancer. JAMA 2004;291:585-90. [Crossref] [PubMed]
- Kinoshita A, Onoda H, Imai N, et al. The Glasgow Prognostic Score, an inflammation based prognostic score, predicts survival in patients with hepatocellular carcinoma. BMC Cancer 2013;13:52. [Crossref] [PubMed]
- Kinoshita A, Onoda H, Imai N, et al. The C-Reactive Protein/Albumin Ratio, a Novel Inflammation-Based Prognostic Score, Predicts Outcomes in Patients with Hepatocellular Carcinoma. Ann Surg Oncol 2015;22:803-10. [Crossref] [PubMed]
- Kim YK, Kim SH, Lee SD, et al. Pretransplant serum levels of C-reactive protein predict prognoses in patients undergoing liver transplantation for hepatocellular carcinoma. Transplant Proc 2015;47:686-93. [Crossref] [PubMed]
- Kornberg A, Witt U, Kornberg J, et al. Postoperative peak serum C-reactive protein is a predictor of outcome following liver transplantation for hepatocellular carcinoma. Biomarkers 2016;21:152-9. [Crossref] [PubMed]
- Li Z, Xue TQ, Chen XY. Predictive values of serum VEGF and CRP levels combined with contrast enhanced MRI in hepatocellular carcinoma patients after TACE. Am J Cancer Res 2016;6:2375-85. [PubMed]
- Rekik S, Guyot E, Bhais M, et al. The CRP level and STATE score predict survival in cirrhotic patients with hepatocellular carcinoma treated by transarterial embolization. Dig Liver Dis 2016;48:1088-92. [Crossref] [PubMed]
- Pinato DJ, Karamanakos G, Arizumi T, et al. Dynamic changes of the inflammation-based index predict mortality following chemoembolisation for hepatocellular carcinoma: A prospective study. Aliment Pharmacol Ther 2014;40:1270-81. [Crossref] [PubMed]
- Fujiwara N, Tateishi R, Nakagawa H, et al. Slight elevation of high-sensitivity C-reactive protein to predict recurrence and survival in patients with early stage hepatitis C-related hepatocellular carcinoma. Hepatol Res 2015;45:645-55. [Crossref] [PubMed]
- Morimoto M, Numata K, Moriya S, et al. Inflammation-based prognostic score for hepatocellular carcinoma patients on sorafenib treatment. Anticancer Res 2012;32:619-23. [PubMed]
- Nakanishi H, Kurosaki M, Tsuchiya K, et al. Novel Pretreatment Scoring Incorporating C-reactive Protein to Predict Overall Survival in Advanced Hepatocellular Carcinoma with Sorafenib Treatment. Liver Cancer 2016;5:257-68. [Crossref] [PubMed]
- Pinato DJ, Stebbing J, Ishizuka M, et al. A novel and validated prognostic index in hepatocellular carcinoma: the inflammation based index (IBI). J Hepatol 2012;57:1013-20. [Crossref] [PubMed]
- Xia W, Ke Q, Wang Y, et al. Predictive value of pre-transplant platelet to lymphocyte ratio for hepatocellular carcinoma recurrence after liver transplantation. World J Surg Oncol 2015;13:60. [Crossref] [PubMed]
- Fan W, Zhang Y, Wang Y, et al. Neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios as predictors of survival and metastasis for recurrent hepatocellular carcinoma after transarterial chemoembolization. PLoS One 2015;10:e0119312. [Crossref] [PubMed]
- Tian XC, Liu XL, Zeng FR, et al. Platelet-to-lymphocyte ratio acts as an independent risk factor for patients with hepatitis B virus-related hepatocellular carcinoma who received transarterial chemoembolization. Eur Rev Med Pharmacol Sci 2016;20:2302-9. [PubMed]
- Xue TC, Jia QA, Ge NL, et al. The platelet-to-lymphocyte ratio predicts poor survival in patients with huge hepatocellular carcinoma that received transarterial chemoembolization. Tumour Biol 2015;36:6045-51. [Crossref] [PubMed]
- Peng W, Li C, Zhu WJ, et al. Prognostic value of the platelet to lymphocyte ratio change in liver cancer. J Surg Res 2015;194:464-70. [Crossref] [PubMed]
- Kaida T, Nitta H, Kitano Y, et al. Preoperative platelet-to-lymphocyte ratio can predict recurrence beyond the Milan criteria after hepatectomy for patients with hepatocellular carcinoma. Hepatol Res 2017;47:991-9. [Crossref] [PubMed]
- Zhao Y, Si G, Zhu F, et al. Prognostic role of platelet to lymphocyte ratio in hepatocellular carcinoma: a systematic review and meta-analysis. Oncotarget 2017;8:22854-62. [PubMed]
- Gay LJ, Felding-Habermann B. Contribution of platelets to tumour metastasis. Nat Rev Cancer 2011;11:123-34. [Crossref] [PubMed]
- Li X, Chen ZH, Xing YF, et al. Platelet-to-lymphocyte ratio acts as a prognostic factor for patients with advanced hepatocellular carcinoma. Tumour Biol 2015;36:2263-9. [Crossref] [PubMed]
Cite this article as: Rosenblatt RE, Tafesh ZH, Halazun KJ. Role of inflammatory markers as hepatocellular cancer selection tool in the setting of liver transplantation. Transl Gastroenterol Hepatol 2017;2:95.


