Therapeutic strategies for wild-type gastrointestinal stromal tumor: is it different from KIT or PDGFRA-mutated GISTs?
Gastrointestinal stromal tumor (GIST) is the most common and potentially-malignant mesenchymal tumor in the gastrointestinal tract (1,2). GIST may arise at any age although it is frequently reported around the 60s. GISTs are found most often in the stomach, followed by the small intestine, but GISTs may occur at any sites of the gastrointestinal tract and peritoneal cavity including the colon and esophagus. The proliferation of most GISTs (80% to 90%) is driven by gain-of-function mutations either in the KIT or platelet-derived growth factor receptor alpha (PDGFRA) gene, which are mutually exclusive. Surgery is the only potentially-curative treatment for primary GIST. Nearly 40% of GIST patients, however, have had disease recurrence even after complete resection (3). Imatinib mesylate (Gleevec, Novartis Pharmaceuticals, Basel, Switzerland) has revolutionized treatment of advanced and recurrent GISTs by inhibiting the KIT and PDGFRA signaling pathways (4).
Wild-type GISTs are usually defined as GISTs which lack mutations both in the KIT and PDGFRA genes (1,2,4). Thus, wild-type GISTs may have several different driver mutations and may be consisted from heterogeneous entities, such as succinate dehydrogenase (SDH)-deficient GISTs, BRAF-mutated GISTs, and neurofibromatosis type I-associated GISTs (NF1-GISTs). Wild-type GISTs may comprise nearly 10% of adult GISTs and may be more commonly found in GISTs affecting children and adolescents. Wild-type GISTs are generally considered to be indolent in clinicopathological features and insensitive to imatinib. However, wild-type GISTs lack details of clinical features, outcomes of surgical and medical treatment.
Recently, Weldon et al. have reported surgical outcomes for wild-type GISTs based on their data obtained from the NIH Pediatric and Wild-Type GIST Clinic (5). They have shown that wild-type GISTs is an indolent disease even after relapses with the median event-free survival (EFS) of 2.5 years and that EFS after primary surgery is significantly related to the presence of metastatic disease and high mitosis (>5/50 HPF), whereas negative microscopic resection margins and type of gastric resection had no significant effects. They also suggested that repeated resection was significantly associated with decreased postoperative EFS compared with initial procedures, and indicated that subsequent resections could be performed only to address symptoms such as obstruction or bleeding. This study has the largest database collecting wild type GISTs. The report significantly contributes understanding of current outcomes of wild-type GIST patients after surgery and its prognostic factors, especially those for SDH-deficient GISTs.
The diagnosis of molecular subtypes of wild-type GISTs is often performed as shown Figure 1 in the clinical practice and research (2,6,7). SDHB-immunostaining discriminates SDH-deficient and SDH-competent GISTs. The former may have either mutation in SDH subunits or promoter methylation of the SDH genes (6-8). The latter may consist from NF1-GISTs and the other GISTs with rare driver mutations, such as, mutations in the BRAF, KRAS, or CBL gene (7,9-13) (Table 1). Incidence of GISTs is estimated to be 10/million/year and GISTs without mutations in KIT and PDGFRA may account for 10–15% of total GISTs. However, the precise incidence of each rare subtype is unknown, although SDH-deficient GIST is the most frequent wild-type GIST. Each molecular subtype may have some specific features including location, clinical presentation and pathological characteristics as shown in Table 1, though, they are based on small retrospective studies (6-13). Wild-type GISTs consistently express KIT tyrosine kinase, however, KIT and PDGFRA tyrosine kinases are, typically, not activated because the kinases lack auto-activation mechanisms, such as gain-of-function mutations or autocrine-loop. Basic and clinical data indicate that imatinib, a KIT/PDGFRA-targeting agent, is not considered to have antitumor activities for “true” wild-type GISTs (18). Recent small cohort studies and sub-analysis of clinical trials indicate that inhibitors for vascular endothelial growth factor receptor, such as sunitinib, regorafenib, and pazopanib, may have significant activities on SDH-deficient GISTs (16-18). GISTs with typical BRAF mutations (V600E) were indicated to be sensitive to BRAF and/or MEK inhibitors (15). Some translational investigations indicate that the proliferation of NF1-GISTs may be potentially controlled by MEK1/2 inhibitors (14). These findings suggest that, in future, medical treatment for wild-type GISTs should be developed depending on their molecular features (18).
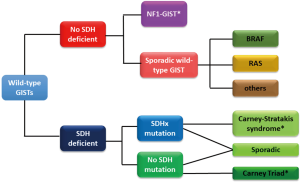
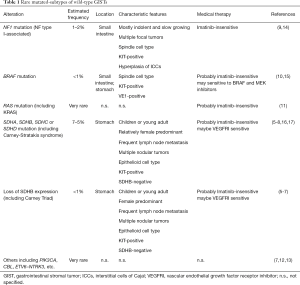
Full table
The study of Weldon et al. (5) predominantly included young adults and pediatric patients, thus, more than half of patients included in the study were SDH-deficient GISTs. From their data, SDH-deficient GISTs had relatively more events (recurrence or progression) after initial surgery than SDH-competent ones, although the statistics are marginal. Each subtype of wild-type GISTs may have individual clinicopathological-features and may show different clinical outcomes (Table 1), for example, NF1-GISTs may have multiple tumors and, sometimes, the NF1 patients may have second-primary GISTs, but not recurrent, after initial complete surgery (9). Hence, it may be thought that the study mainly reflected surgical outcomes and prognostic factors of SDH-deficient GISTs and it may not be always true for the other rare molecular-subtype GISTs. The other thing is extent of organ resection. Most GIST guidelines have suggested to consider preservation of organ function at surgical resection from early on, which has strongly encouraged surgeons to avoid anatomic resection and adopt partial or wedge resection when applicable (2,19-21). Accordingly, most anatomic resection, such as distal or total gastrectomy, was probably unavoidable for complete resection due to tumor extent including large tumor size, multiple occurrence, and/or nodular extension, although the authors recommended a limited surgery with wedge resection for wild-type GISTs. They compared surgical outcomes after primary and subsequent operations, and found significant difference between them. They have not evaluated treatment with interventional radiology, such as radiofrequency ablation (RFA), cryoablation and/or transarterial embolization (TAE), which are less invasive than surgery but are still required evidence for recurrent or imatinib-resistant GISTs (1,2).
In summary, wild-type GISTs account for 10% to 15% of total GISTs and still lack evidence-based treatment strategies. The several reports including Weldon et al. suggest that, compared with KIT or PDGFRA-mutated GISTs, wild-type GISTs are generally indolent in clinicopathological features and show relatively good prognosis even after recurrence (5-13). Limited resection, taking account of organ-function, is recommended for primary wild-type GIST, and roles of surgery are limited for recurrent disease of wild-type GIST. Weldon et al. (5) also indicated that watch-and-wait approach may be applicable for wild-type GISTs when biology is indolent. Wild-type GISTs are heterogeneous entity and consist of several molecular subtypes, including GISTs with mutations in SDH genes, those lacking expression of SDH complex without mutations, those associated with NF1, those with mutations in the BRAF, KRAS or other genes. They are considered to be insensitive to imatinib, and therapeutic agents will be developed based on their molecular features. The work of Weldon et al. (5) has opened the evidence-door of surgical treatment for wild-type GIST, and we still need more evidences based on clinical studies and on prospective real-world registry research because it is extremely rare.
Acknowledgements
Funding: This work is supported in part by a Grant-in-Aid (16H05419 and 16K15600) for Scientific Research from the Japanese Ministry of Education, Culture, Sports, Science and Technology, by a Grant (28-A-16) from the National Cancer Center Research and Development Fund.
Footnote
Conflicts of Interest: T Nishida has received honoraria for speeches from Novartis, Bayer, Eisai, and Pfizer.
References
- Joensuu H, Hohenberger P, Corless CL. Gastrointestinal stromal tumour. Lancet 2013;382:973-83. [Crossref] [PubMed]
- Nishida T, Blay JY, Hirota S, et al. The standard diagnosis, treatment, and follow-up of gastrointestinal stromal tumors based on guidelines. Gastric Cancer 2016;19:3-14. [Crossref] [PubMed]
- Joensuu H, Vehtari A, Riihimäki J, et al. Risk of recurrence of gastrointestinal stromal tumour after surgery: an analysis of pooled population-based cohorts. Lancet Oncol 2012;13:265-74. [Crossref] [PubMed]
- Demetri GD, von Mehren M, Antonescu CR, et al. NCCN Task Force report: update on the management of patients with gastrointestinal stromal tumors. J Natl Compr Canc Netw 2010;8 Suppl 2:S1-41. [Crossref] [PubMed]
- Weldon CB, Madenci AL, Boikos SA, et al. Surgical Management of Wild-Type Gastrointestinal Stromal Tumors: A Report From the National Institutes of Health Pediatric and Wildtype GIST Clinic. J Clin Oncol 2016. [Epub ahead of print]. [PubMed]
- Janeway KA, Kim SY, Lodish M, et al. Defects in succinate dehydrogenase in gastrointestinal stromal tumors lacking KIT and PDGFRA mutations. Proc Natl Acad Sci U S A 2011;108:314-8. [Crossref] [PubMed]
- Boikos SA, Pappo AS, Killian JK, et al. Molecular Subtypes of KIT/PDGFRA Wild-Type Gastrointestinal Stromal Tumors: A Report from the National Institutes of Health Gastrointestinal Stromal Tumor Clinic. JAMA Oncol 2016;2:922-8. [Crossref] [PubMed]
- Killian JK, Kim SY, Miettinen M, et al. Succinate dehydrogenase mutation underlies global epigenomic divergence in gastrointestinal stromal tumor. Cancer Discov 2013;3:648-57. [Crossref] [PubMed]
- Nishida T, Tsujimoto M, Takahashi T, et al. Gastrointestinal stromal tumors in Japanese patients with neurofibromatosis type I. J Gastroenterol 2016;51:571-8. [Crossref] [PubMed]
- Huss S, Pasternack H, Ihle MA, et al. Clinicopathological and molecular features of a large cohort of gastrointestinal stromal tumors (GISTs) and review of the literature: BRAF mutations in KIT/PDGFRA wild-type GISTs are rare events. Hum Pathol 2017;62:206-14. [Crossref] [PubMed]
- Hechtman JF, Zehir A, Mitchell T, et al. Novel oncogene and tumor suppressor mutations in KIT and PDGFRA wild type gastrointestinal stromal tumors revealed by next generation sequencing. Genes Chromosomes Cancer 2015;54:177-84. [Crossref] [PubMed]
- Brenca M, Rossi S, Polano M, et al. Transcriptome sequencing identifies ETV6-NTRK3 as a gene fusion involved in GIST. J Pathol 2016;238:543-9. [Crossref] [PubMed]
- Lasota J, Felisiak-Golabek A, Wasag B, et al. Frequency and clinicopathologic profile of PIK3CA mutant GISTs: molecular genetic study of 529 cases. Mod Pathol 2016;29:275-82. [Crossref] [PubMed]
- Jessen WJ, Miller SJ, Jousma E, et al. MEK inhibition exhibits efficacy in human and mouse neurofibromatosis tumors. J Clin Invest 2013;123:340-7. [Crossref] [PubMed]
- Falchook GS, Trent JC, Heinrich MC, et al. BRAF mutant gastrointestinal stromal tumor: first report of regression with BRAF inhibitor dabrafenib (GSK2118436) and whole exomic sequencing for analysis of acquired resistance. Oncotarget 2013;4:310-5. [Crossref] [PubMed]
- Mir O, Cropet C, Toulmonde M, et al. Pazopanib plus best supportive care versus best supportive care alone in advanced gastrointestinal stromal tumours resistant to imatinib and sunitinib (PAZOGIST): a randomised, multicentre, open-label phase 2 trial. Lancet Oncol 2016;17:632-41. [Crossref] [PubMed]
- Maki RG, Blay JY, Demetri GD, et al. Key Issues in the Clinical Management of Gastrointestinal Stromal Tumors: An Expert Discussion. Oncologist 2015;20:823-30. [Crossref] [PubMed]
- Nishida T, Doi T, Naito Y. Tyrosine kinase inhibitors in the treatment of unresectable or metastatic gastrointestinal stromal tumors. Expert Opin Pharmacother 2014;15:1979-89. [Crossref] [PubMed]
- Nishida T, Hirota S, Yanagisawa A, et al. Clinical practice guideline for gastrointestinal stromal tumor (GIST) in Japan. Int J Clin Oncol 2008;13:416-30. [Crossref] [PubMed]
- Koo DH, Ryu MH, Kim KM, et al. Asian Consensus Guidelines for the Diagnosis and Management of Gastrointestinal Stromal Tumor. Cancer Res Treat 2016;48:1155-66. [Crossref] [PubMed]
- ESMO/European Sarcoma Network Working Group. Gastrointestinal stromal tumours: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2014;25 Suppl 3:iii21-6. [Crossref] [PubMed]
Cite this article as: Nishida T. Therapeutic strategies for wild-type gastrointestinal stromal tumor: is it different from KIT or PDGFRA-mutated GISTs? Transl Gastroenterol Hepatol 2017;2:92.


