Bridging patients with hepatocellular cancer waiting for liver transplant: all the patients are the same?
Hepatocellular carcinoma (HCC) is the fifth more frequent cancer worldwide and the most common primary liver tumor. Liver transplant (LT) is considered the best curative treatment for patients with cirrhosis and HCC within Milan criteria (1 tumor ≤5 cm and up to 3 tumors ≤3 cm). It removes all the liver affected by cancer and at the same time it treats the underlying liver disease, with a survival rate of 70% and a 5 years recurrence rate of less than 20%. Unfortunately, the applicability of LT for HCC patients is limited by the shortage of liver grafts, determining a longer time on waitlist and high dropout rate. Bridging treatments are neo-adjuvant antitumoral therapies given to patients on the waitlist for LT, affected by HCC within the criteria, with the aim to reduce the disease progression and therefore the dropout rate. Indeed, these treatments act as a “bridge” until a suitable donor organ becomes available. Most of bridging therapies are locoregional treatments (LRTs). Dropout rate for HCC progression increases in a time-dependent way (1) and evidences show higher dropout rates in patients with tumor >3 cm and an expected waiting time longer than 3–6 months (2). In different reports, if HCC is left untreated, the risk of drop out at 6 months and at 1 year has been estimated to be respectively 12% and 15–30% (3,4). Risk factors for dropout include: tumor diameter greater than 3 cm or multifocal disease, serum α-fetoprotein (AFP) level greater than 200 ng/mL, waiting time longer than 6 months and lack of response to bridging therapy (5). Although there are no randomized control trials (RCT) evaluating the efficacy of neo-adjuvant therapies in reducing dropout rate and improving survival after LT, LRT is accepted as the standard of care for patients expected to stay on the waitlist for more than 6 months. In patients with HCC within Milan criteria, bridging therapy is estimated to reduce dropout rate to 0–10%. A retrospective study assessed the effectiveness of neo-adjuvant treatment in decreasing dropout rate and found that the 49% of patients with a complete response to LRT had a significant reduction in dropout at 3, 6, and 12 months (6). Other studies showed that bridge therapies are successful in keeping patients on the waitlist and they increase the likelihood of LT, specifically in longer waitlist time (7,8). The currently available evidence about survival benefit in HCC patients receiving pre-transplant LRT remains heterogeneous and contradictory. Even if some evidences indicate that bridging therapy can increase post-transplant survival rate (9), this statement is not confirmed by a recent large multicentric analysis. This retrospective study evaluated the impact of LRT on recurrence and survival after LT on 3,601 recipients with HCC within Milan criteria and did not demonstrate any advantage in terms of survival benefit and recurrence free survival (RFS) in patients treated with LRT, compared to patients not receiving LRT at all (10). The effectiveness of bridging LRT on improving post-transplant overall survival (OS) and RFS is limited to patients with a complete pathologic response of the tumor after LRT (10,11). To reduce dropout from the waitlist for tumor progression in HCC patients awaiting LT, a consensus statement recommends that bridging therapies should be considered for patients with 1 nodule of 2–5 cm or up to 3 nodules each ≤3 cm, expected to wait longer than 6 months to reduce dropout from the waiting list because of tumor progression. Different modalities have been proposed as bridging therapies, the most common is transarterial chemoembolization (TACE). Currently, any survival benefit has been demonstrated for any particular LRT modality, so no one form of treatment can be recommended over another (10,12).
Liver resection (LR)
LR is commonly used as primary curative treatment for HCC. OS after LR in cirrhotic patients is over 50% at 5 years and perioperative mortality is 2–3% (13,14). LR can be considered as a first line bridging treatment to LT. The theoretical advantage of surgery is a better control of tumor growth, as TACE and other LRT do not achieve complete tumor ablation as well as surgery. Moreover, the pathologic analysis of the resected specimen allows an evaluation of tumor biology and provides a selection of patients with risk factors of poor prognosis who are at major risk of early recurrence and should have a priority in LT waitlist (15). However, in most transplant Centers, for HCC patients waiting for LT, TACE and other LRT are preferred, mainly because LR, compared to non-surgical therapies, has higher costs and more complications and can only be performed in well-compensated liver disease.
TACE
TACE is the most widely used bridging treatment. A chemotherapeutic drug (commonly doxorubicin, cisplatin or mitomycin C), emulsified in lipiodol with embolizing material, is injected into the branch of the hepatic artery feeding the tumor, with the aim to induce hypoxemia and tumor necrosis. This technique has been enhanced by the introduction of drug-eluting beads (DEB-TACE), with higher dose and retention of chemotherapeutic drug into the tumor and reduction of systemic toxicity. In the histological examination, TACE achieves complete pathological response in less than 30% of cases. Many authors analysed the impact of TACE as bridging therapy to LT on dropout rates in waitlist, survival and recurrence after LT. The reported results of bridging therapy with TACE are controversial and no prospective RCTs have confirmed its efficacy in reducing dropout rates. Many authors demonstrated that a good response to TACE (necrosis >60%) is significantly related to an improved long-term survival after LT and a lower recurrence rate (16). Others did not find any significant advantage in overall and RFS after LT in HCC patients bridged with TACE (17,18). Despite various reports had suggested favorable long-term outcome in patients successfully bridged with TACE, the real benefit in terms of survival after LT remains questionable, nevertheless TACE remains a widely used technique in clinical practice.
Radiofrequency ablation (RFA)
RFA is an ablative technique that uses a radiofrequency electrode tip generating alternating current, that induces coagulative necrosis in the target tumor by thermal action, with temperatures of 60 to 100 °C. It can be performed by intraoperative or percutaneous approach. RFA is known to be an effective curative treatment for patients with non-resectable HCC. When used as a bridging treatment, RFA reduces significantly the dropout rate (19). The success in achieving complete necrosis depends on the size of the target lesion: RFA for HCC with diameter of 2.5 cm or less lead to complete necrosis in up to 90% of cases. For lesions of 5 cm diameter or more, the remarkable necrotic effect is estimated less than 50% (19). Even if RFA is proven to be a safe procedure, it has some limitations and complications. It should be avoided in subcapsular HCC and in nodules located near bowel loops or gallbladder and the tumor should be visualized by ultrasound. The ‘heat-sink’ effect may reduce RFA efficacy for tumors near the major vessels. Complications of RFA include thermal or mechanical damage, leading to rare but severe complications, such as acute liver failure, liver abscess, haemobilia. Tumor seeding is reported as a very rare complication (0.3–0.5%) (18).
Percutaneous ethanol injection (PEI)
PEI is the oldest technique for the percutaneous ablation of HCC, introduced in the 1980s to treat small HCC safely and effectively. At present, PEI is rarely used as bridging technique, while thermal ablation procedures [RFA or microwave ablation (MWA)] are currently preferred, because they need a small number of treatments and give better tumor control (20,21).
MWA
MWA is a percutaneous thermal ablation procedure that has been shown to be effective and promising as bridge therapy of HCC. As well as RFA, the lesion should be visualized by ultrasound for an exact localization. Compared to RFA, MWA leads to a larger volume of necrosis and it seems to be more effective in multifocal disease and for nodules located near large vessels, because of the lack of ‘heat sink’ effect. Many authors reported similar response rate with RFA (22,23) and a clear advantage of MWA versus RFA has not been demonstrated.
Irreversible electroporation (IRE)
IRE is a non-thermal ablative technique that uses high-voltage electricity to induce apoptosis of target cells by increasing irreversibly the membrane permeability. It also induces complete cell death even in lesions adjacent to large vessels, without the ‘heat sink’ effect seen in RFA. IRE should have a potential role in patients in waitlist, but currently there are few data about his use as bridging therapy. Cheng et al. report a high rate of complete necrosis for IRE used in the treatment of tumor <3 cm (24).
Transarterial radioembolization (TARE)
TARE is an intra-arterial therapy using microspheres coated with Yttrium-90 (Y90). This technique allows a high concentration of radioactive substance in the lesion, with minimum toxic damage to the surrounding liver parenchyma. It is also a safe procedure in case of portal vein thrombosis (25). Most of the current studies are focused on TARE used as downstaging therapy for patients with HCC out of the criteria, then there are not many studies about the role of TARE in bridging therapy. Compared with TACE, TARE seems to allow a good tumor response in shorter times and a longer time to progression, suggesting a potential advantage in bridging therapy. TARE is not indicated for all patients: before the procedure an assessment of the vascular anatomy is required and a mesenteric angiogram with 99Tc macroaggregated albumin should be performed.
High intensity focused ultrasound (HIFU)
HIFU is a totally extracorporeal ablative technique, that uses ultrasound beams to induce heat reaction, reaching 60 °C of temperature or more, leading coagulative necrosis in the HCC nodule. The heat damage to the tissues between the transducer and the target is reduced to minimum. Cheung et al. described the experience of HIFU used as a bridging therapy and observed an improvement of the rate of patients receiving bridging therapy in the waiting list and a reduction of drop-out rate (26,27). Further researches are needed to assess the real survival benefit after the LT in patients previously treated with HIFU. Before performing HIFU, it is necessary to assess the localization of the nodule by ultrasound. It is a safe procedure with minimal risk. Very rare but severe complications, such as bile duct injury, have been reported; minor complications such as skin and subcutaneous tissue injuries are described in many patients(28).
Stereotactic body radiotherapy (SBRT)
SBRT is an extracorporeal technique consisting in a high dose of radiations focused on the target lesion, needing few treatment sessions. It is an alternative bridging therapy for patients with decompensated liver function that would not be candidate to other bridging therapy (29). Data regarding the use of SBRT as a bridging treatment are scarce. Sapisochin et al. recently reported an intention to treat analysis about SBRT used as a bridging therapy in patient not eligible for other LRTs and observed similar drop-out rate with SBRT and RFA or TACE (30). It is proven to be a safe procedure for lesions <6 cm of diameter, even in those localized near the central biliary system, where surgery or ablation cannot be performed (31,32).
Sorafenib
Sorafenib is an oral multi-kinase inhibitor, it delays the tumor progression by inhibiting angiogenesis. The significant efficacy of sorafenib in extending the time-to-progression in patients with advanced HCC is well demonstrated. Studies about Sorafenib used in bridging setting are limited. His effect as neo-adjuvant therapy has been often studied in association with TACE. TACE allows embolization of the tumor feeding artery and it leads to necrosis by local chemotherapeutic effect, in the same time sorafenib inhibit angiogenesis to delay the tumor progression and relapse. The use of sorafenib in combination with other bridging techniques have been described in clinical trials (33), even if the role of combination of bridging therapies is still to be determined.
Choosing the right treatment for each patient
Table 1 summarizes the main characteristics, indications and disadvantages of the different bridging techniques. Any treatment for HCC should be performed with the aim to reduce to minimum the risk of hepatic failure. Up to date, there are no RCT comparing their efficacy in the setting of LT and no guidelines are available to define which patients should receive bridging therapy. Currently, the choice is mainly based on Centre experience. Although the operator’s practice has its importance, the method selected for bridging therapy has to be tailored on the conditions of the patient, considering also tumor features and stage. Basing on BCLC algorithm, different treatments are suggested.
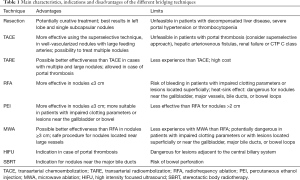
Full table
Patients in very early stage (BCLC-0): good liver function (Child-Pugh A), single nodule less than 2 cm, without vascular invasion neither satellitosis (HCC T1 in TNM classification). These patients have an excellent outcome: 5-year survival after resection or transplantation is 80–90% and 70% after LRT. The risk of recurrence at 3 years is 3% (34). The choice of the bridging technique depends on portal hypertension and localization of the nodule. If MELD score is less than 10 and there is not portal hypertension, without thrombocytopenia, LR is the treatment of choice. In fact, LR can only be offered to selected patients. Single subcapsular or exophytic HCC, or tumors in the left lobe are the best tumors to be treated with LR in bridging setting. There is no significant difference in post-operative complications and 3- and 5-year OS between cirrhotic HCC patients undergoing primary LT or secondary LT after LR (35). If performing LR is not possible, RFA, PEI or IRE can be considered, even if Clavien et al. assess the lack of evidence of usefulness of bridging treatments in patients with T1 HCC (12). Patients in early stage (BCLC-A): Child-Pugh class A or B, with single nodule or less than 3 nodules, each one with a diameter of 3 cm or less. In this category of patients bridging therapies mainly consist in LRT. RFA should be preferred in patients with single nodule less than 5 cm. The best effect of RFA as bridging therapy is shown in patients with small tumors (3 cm or smaller) with a waitlist time of less than 1 year (36). PEI seems to have lower efficacy than RFA and can be used in small HCC located in sites considered dangerous for RFA (for example near the bowel loops or the gallbladder). TACE is indicated in patients with one or more nodules and should be considered the treatment of choice for HCC between 3–5 cm, because nodules with 3 cm of diameter or more are better vascularized, with a large feeding artery, therefore the effectiveness of TACE appears to be better; whereas smaller HCC have not yet a completely developed arterial neoangiogenesis (14,37). In this class of patients, TARE should also be considered and some authors assess the potential advantage of this technique towards TACE, because it seems to need less treatments and the recurrence time is longer (38). Patients with ascites: even if BCLC algorithm does not indicate TACE for treatment of HCC, it has been considered anyway, in selected patients in waitlist, only if performed in superselective way. In case of portal thrombosis, TACE has always been considered not indicated, for the high risk of hepatic failure, but other studies have shown his feasibility as bridge therapy, only if superselective. In these patients TARE has been indicated as the better choice, it is described as a safer procedure, because it keeps minimum toxicity to the functional liver parenchyma. In patients in waitlist with HCC and Child-Pugh C cirrhosis, TACE is not indicated. Bridging therapy can be safely performed with TARE or HIFU, that had been shown to lead to good radiological responses, with minimum risk of worsening liver function (27). Every technique has his collateral effect towards liver parenchyma, biliary tree, venous structures of adjacent organs and this must be considered in the choice of the treatment. The presence of the “heath sink effect” in RFA can lead to incomplete ablation of nodules near the major vessels. RFA may be dangerous for lesions near the gallbladder or bowel, for the risk of biliary lesions, hemorrhage or gastrointestinal perforation. For lesions near the gallbladder or bowel, PEI seems to be safer than RFA. In the same way, SBRT is not indicated for lesions near the bowel, for his risk of bleeding and perforation (31). However, SBRT have the advantage to treat the lesions adjacent to the central biliary system, that are not suitable for LR or ablation. For lesions near major vessels, where RFA is contraindicated, MWA could be a safe treatment option (39).
Conclusions
Different techniques are currently in use as bridging therapies for LT, in order to decrease drop-out rate from waitlist. The selection of each therapy is based on liver function and tumor features, like localization, dimensions and number of nodules. Further studies have to be performed to assess the efficacy and feasibility of many of these neo-adjuvant therapies.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Yao FY, Ferrell L, Bass NM, et al. Liver transplantation for hepatocellular carcinoma: comparison of the proposed UCSF criteria with the Milan criteria and the Pittsburgh modified TNM criteria. Liver Transpl 2002;8:765-74. [Crossref] [PubMed]
- Pomfret EA, Washburn K, Wald C, et al. Report of a national conference on liver allocation in patients with hepatocellular carcinoma in the United States. Liver Transpl 2010;16:262-78. [Crossref] [PubMed]
- Washburn K, Edwards E, Harper A, et al. Hepatocellular carcinoma patients are advantaged in the current liver transplant allocation system. Am J Transplant 2010;10:1643-8. [Crossref] [PubMed]
- Yao FY, Bass NM, Nikolai B, et al. Liver transplantation for hepatocellular carcinoma: analysis of survival according to the intention-to-treat principle and dropout from the waiting list. Liver Transpl 2002;8:873-83. [Crossref] [PubMed]
- Yao FY, Bass NM, Nikolai B, et al. A follow-up analysis of the pattern and predictors of dropout from the waiting list for liver transplantation in patients with hepatocellular carcinoma: implications for the current organ allocation policy. Liver Transpl 2003;9:684-92. [Crossref] [PubMed]
- Cucchetti A, Cescon M, Bigonzi E, et al. Priority of candidates with hepatocellular carcinoma awaiting liver transplantation can be reduced after successful bridge therapy. Liver Transpl 2011;17:1344-54. [Crossref] [PubMed]
- Mehta N, Dodge JL, Goel A, et al. Identification of liver transplant candidates with hepatocellular carcinoma and a very low dropout risk: implications for the current organ allocation policy. Liver Transpl 2013;19:1343-53. [Crossref] [PubMed]
- Sheth RA, Patel MS, Koottappillil B, et al. Role of Locoregional Therapy and Predictors for Dropout in Patients with Hepatocellular Carcinoma Listed for Liver Transplantation. J Vasc Interv Radiol 2015;26:1761-8; quiz 8.
- Terzi E, Golfieri R, Piscaglia F, et al. Response rate and clinical outcome of HCC after first and repeated cTACE performed "on demand". J Hepatol 2012;57:1258-67. [Crossref] [PubMed]
- Agopian VG, Harlander-Locke MP, Ruiz RM, et al. Impact of Pretransplant Bridging Locoregional Therapy for Patients With Hepatocellular Carcinoma Within Milan Criteria Undergoing Liver Transplantation: Analysis of 3601 Patients from the US Multicenter HCC Transplant Consortium. Ann Surg 2017;266:525-35. [Crossref] [PubMed]
- Chan KM, Yu MC, Chou HS, et al. Significance of tumor necrosis for outcome of patients with hepatocellular carcinoma receiving locoregional therapy prior to liver transplantation. Ann Surg Oncol 2011;18:2638-46. [Crossref] [PubMed]
- Clavien PA, Lesurtel M, Bossuyt PM, et al. Recommendations for liver transplantation for hepatocellular carcinoma: an international consensus conference report. Lancet Oncol 2012;13:e11-22. [Crossref] [PubMed]
- Chang YJ, Chung KP, Chang YJ, et al. Long-term survival of patients undergoing liver resection for very large hepatocellular carcinomas. Br J Surg 2016;103:1513-20. [Crossref] [PubMed]
- European Association For The Study Of The L. European Organisation For R, Treatment Of C. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 2012;56:908-43. [Crossref] [PubMed]
- Sala M, Fuster J, Llovet JM, et al. High pathological risk of recurrence after surgical resection for hepatocellular carcinoma: an indication for salvage liver transplantation. Liver Transpl 2004;10:1294-300. [Crossref] [PubMed]
- Allard MA, Sebagh M, Ruiz A, et al. Does pathological response after transarterial chemoembolization for hepatocellular carcinoma in cirrhotic patients with cirrhosis predict outcome after liver resection or transplantation? J Hepatol 2015;63:83-92. [Crossref] [PubMed]
- Decaens T, Roudot-Thoraval F, Bresson-Hadni S, et al. Impact of pretransplantation transarterial chemoembolization on survival and recurrence after liver transplantation for hepatocellular carcinoma. Liver Transpl 2005;11:767-75. [Crossref] [PubMed]
- She WH, Cheung TT. Bridging and downstaging therapy in patients suffering from hepatocellular carcinoma waiting on the list of liver transplantation. Transl Gastroenterol Hepatol 2016;1:34. [Crossref] [PubMed]
- Pompili M, Mirante VG, Rondinara G, et al. Percutaneous ablation procedures in cirrhotic patients with hepatocellular carcinoma submitted to liver transplantation: Assessment of efficacy at explant analysis and of safety for tumor recurrence. Liver Transpl 2005;11:1117-26. [Crossref] [PubMed]
- Branco F, Bru C, Vilana R, et al. Percutaneous ethanol injection before liver transplantation in the hepatocellular carcinoma. Ann Hepatol 2009;8:220-7. [PubMed]
- Castroagudin JF, Delgado M, Villanueva A, et al. Safety of percutaneous ethanol injection as neoadjuvant therapy for hepatocellular carcinoma in waiting list liver transplant candidates. Transplant Proc 2005;37:3871-3. [Crossref] [PubMed]
- Boutros C, Somasundar P, Garrean S, et al. Microwave coagulation therapy for hepatic tumors: review of the literature and critical analysis. Surg Oncol 2010;19:e22-32. [Crossref] [PubMed]
- Lu MD, Xu HX, Xie XY, et al. Percutaneous microwave and radiofrequency ablation for hepatocellular carcinoma: a retrospective comparative study. J Gastroenterol 2005;40:1054-60. [Crossref] [PubMed]
- Cheng RG, Bhattacharya R, Yeh MM, et al. Irreversible Electroporation Can Effectively Ablate Hepatocellular Carcinoma to Complete Pathologic Necrosis. J Vasc Interv Radiol 2015;26:1184-8. [Crossref] [PubMed]
- Inarrairaegui M, Pardo F, Bilbao JI, et al. Response to radioembolization with yttrium-90 resin microspheres may allow surgical treatment with curative intent and prolonged survival in previously unresectable hepatocellular carcinoma. Eur J Surg Oncol 2012;38:594-601. [Crossref] [PubMed]
- Cheung TT, Fan ST, Chan SC, et al. High-intensity focused ultrasound ablation: an effective bridging therapy for hepatocellular carcinoma patients. World J Gastroenterol 2013;19:3083-9. [Crossref] [PubMed]
- Chok KS, Cheung TT, Lo RC, et al. Pilot study of high-intensity focused ultrasound ablation as a bridging therapy for hepatocellular carcinoma patients wait-listed for liver transplantation. Liver Transpl 2014;20:912-21. [Crossref] [PubMed]
- Cheung TT, Chu FS, Jenkins CR, et al. Tolerance of high-intensity focused ultrasound ablation in patients with hepatocellular carcinoma. World J Surg 2012;36:2420-7. [Crossref] [PubMed]
- Kollmann D, Selzner N, Selzner M. Bridging to liver transplantation in HCC patients. Langenbecks Arch Surg 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Sapisochin G, Barry A, Doherty M, et al. Stereotactic body radiotherapy vs. TACE or RFA as a bridge to transplant in patients with hepatocellular carcinoma. An intention-to-treat analysis. J Hepatol 2017;67:92-9. [Crossref] [PubMed]
- Eriguchi T, Takeda A, Sanuki N, et al. Acceptable toxicity after stereotactic body radiation therapy for liver tumors adjacent to the central biliary system. Int J Radiat Oncol Biol Phys 2013;85:1006-11. [Crossref] [PubMed]
- Sandroussi C, Dawson LA, Lee M, et al. Radiotherapy as a bridge to liver transplantation for hepatocellular carcinoma. Transpl Int 2010;23:299-306. [Crossref] [PubMed]
- Hoffmann K, Glimm H, Radeleff B, et al. Prospective, randomized, double-blind, multi-center, Phase III clinical study on transarterial chemoembolization (TACE) combined with Sorafenib versus TACE plus placebo in patients with hepatocellular cancer before liver transplantation - HeiLivCa BMC Cancer 2008;8:349. [ISRCTN24081794]. [Crossref] [PubMed]
- Llovet JM, Bruix J. Novel advancements in the management of hepatocellular carcinoma in 2008. J Hepatol 2008;48 Suppl 1:S20-37. [Crossref] [PubMed]
- Belghiti J, Cortes A, Abdalla EK, et al. Resection prior to liver transplantation for hepatocellular carcinoma. Ann Surg 2003;238:885-92; discussion 92-3. [Crossref] [PubMed]
- Belghiti J, Carr BI, Greig PD, et al. Treatment before liver transplantation for HCC. Ann Surg Oncol 2008;15:993-1000. [Crossref] [PubMed]
- Golfieri R, Cappelli A, Cucchetti A, et al. Efficacy of selective transarterial chemoembolization in inducing tumor necrosis in small (<5 cm) hepatocellular carcinomas. Hepatology 2011;53:1580-9. [Crossref] [PubMed]
- Salem R, Lewandowski RJ, Kulik L, et al. Radioembolization results in longer time-to-progression and reduced toxicity compared with chemoembolization in patients with hepatocellular carcinoma. Gastroenterology 2011;140:497-507 e2.
- Lubner MG, Brace CL, Hinshaw JL, et al. Microwave tumor ablation: mechanism of action, clinical results, and devices. J Vasc Interv Radiol 2010;21:S192-203. [Crossref] [PubMed]
Cite this article as: Coletta M, Nicolini D, Benedetti Cacciaguerra A, Mazzocato S, Rossi R, Vivarelli M. Bridging patients with hepatocellular cancer waiting for liver transplant: all the patients are the same? Transl Gastroenterol Hepatol 2017;2:78.


