Living donor liver transplantation for hepatocellular cancer: an (almost) exclusive Eastern procedure?
Introduction
Hepatocellular carcinoma (HCC) is the fifth most prevalent cancer and third most common cause of cancer-related mortality worldwide (1-3). In 70–90% of all cases, HCC develops in patients with chronic liver disease (4), being the leading cause of death among cirrhotic patients (5). Therefore, liver transplantation (LT) is the best curative treatment modality, since it can cure simultaneously the underlying liver disease and the HCC.
There are many important factors to determine the success of the procedure, but two of them are crucial: tumor’s staging and timing to treatment. HCC stage is directly correlated with the risk of recurrence. MC (one HCC of 5 cm or smaller, or up to three nodules of 3 cm or smaller, without vascular invasion or extrahepatic spread) (6) is the benchmark for patient selection for LT (7,8). In this context, LT confers optimal outcomes, achieving up to 91% 1-year survival in patients within MC. However, intention-to-treat (ITT) rates are substantially lower, mainly due dropout. Llovet et al. (9) identified 23% dropout rate of patients with HCC in the waiting list for a deceased donor LT (DDLT). An option to reduce drop out is to perform LT sooner, which is extremely more feasible in the living donor liver transplantation (LDLT) setting. Another advantage of LDLT is that it can be performed independently from tumor´s staging, enabling liver transplants to patients outside the restrict rules used to include patients with HCC in the DDLT waiting list.
The first LDLT was performed in 1988 at our Institution, in Brazil, a Western country (10). Since then, several surgical and radiologic innovations have established LDLT as a tool for overcoming the shortage of deceased donors. Historically, Western countries adopted LDLT mainly for pediatric patients and limited LDLT indications for adult’s recipients. Interestingly, LDLT was exceptionally well accepted in Eastern countries where liver grafts from deceased donors are extremely scarce and up to 96% patients receive hepatic grafts from living donors (11).
This study aims to compare LDLT performed for HCC patients in Western and Eastern countries.
Methods
Literature review
For this review, a search was conducted with PubMed/MEDLINE database with a combination of the following entry terms: living-donor living transplantation, LDLT, hepatocellular carcinoma and HCC. Articles were restricted to the English language. We focused in studies reporting outcomes of LDLT for HCC. In case some of them contained updated data from previous series, only the most complete study was included in the analysis. Data from each center was encompassed to produce global results of Eastern and Western countries. Another search in the same database was performed with the entry terms: hepatocellular carcinoma, HCC, selection criteria and liver transplantation to recover articles reporting expanded selection criteria beyond Milan.
Data collection from Sao Paulo University Medical School
We also conducted a retrospective study of the data from our institution regarding LDLT cases for HCC from January 2003 to May 2017. Patients’ characteristics and outcomes were extracted from medical charts. In our center, HCC cases are evaluated for LDLT on an individual basis, irrespective of tumor size or number of nodules. Biological variables such as serum AFP level, response to ablation therapy, patients’ performance status, position in deceased-donors waitlist (when within MC) and donor’s and recipient’s opinion about the transplant scenario are all taken into account. The only absolute contra-indication is extra-hepatic disease. The results obtained in our series were added to the Western countries data.
Results
A total of 30 articles (Table 1) were selected for reporting LDLT outcome data and divided according to the geographical location of the reporting center in Eastern or Western countries. Most articles were from Eastern centers and they also encompassed a larger amount of cases (3,854 vs. 457). The recurrence rates found in each region presented, however, a similar distribution. Interestingly, the proportion of cases outside MC was also similar between regions, being the largest one from our institution.
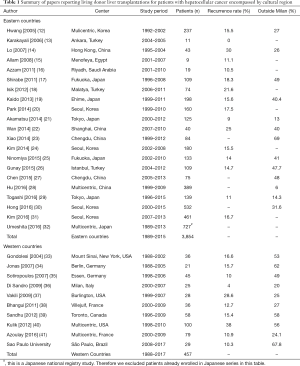
Full table
The most relevant selection criteria for LT in HCC found in literature are presented in Table 2 (DDLT context) and Table 3 (LDLT context). It is noteworthy that while most criteria for DDLT were conceived in the West (87.5%), all criteria for LDLT were created in the East.
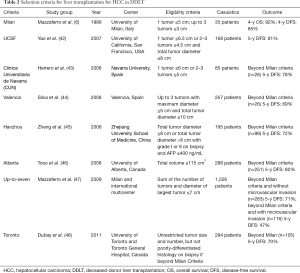
Full table

Full table
Discussion
In 2015, Brazil performed 1,805 LT. Even though LDLT represented only 8.5% of all transplants, it corresponded to 70% of cases in the pediatric population (51). This is probably the usual scenario in others Western countries as well, where LDLT is performed only as an auxiliary option to address graft shortage. This supporting hole of LDLT in Western countries could explain the impressive difference between the numbers of LDLT performed for HCC patients in West and East, as illustrated in Table 1. In the West, most countries already present a well-established program of brain-dead donors and some also allow the use of organs from DCD (donor after cardiac death). Therefore, LDLT is mainly used a complementary tool to overcome organ shortage. On the other side, in many Eastern countries, deceased-donors are extremely scarce due to cultural issues.
In recent years, however, the number of LDLT is growing in Western countries, especially for HCC patients. The main reason is that LDLT offers a valuable opportunity of drastically reducing the waiting time for transplantation. Thereby, it diminishes not only waitlist mortality but also dropout rates due to tumor progression beyond established criteria for DDLT, which usually are very restrictive. The most common worldwide is the MC (6). Patients within MC present a 5-year overall survival (OS) of 65% to 78%. These numbers are particularly encouraging when compared to 5-year survival rates of non-HCC transplanted patients, which range from 68 to 87 (52).
Unfortunately, MC is also over-restrictive and many patients when diagnosed with HCC are already outside it. Even employing annual surveillance in cirrhotics by means of liver ultrasound combined with serum levels of alfa-fetoprotein measurement, HCC can be discovered beyond MC in 20% of the patients (53). That is the reason why expanded criteria have been proposed throughout the last decade. The most relevant of them are mentioned in Table 2. Despite increasing the number of patients suitable for LT, these criteria present outcomes comparable to patients within MC.
Most expanded criteria modify the number and size of nodules originally determined in MC. Nonetheless, apart from and gross pathologic tumor features (size, number and location of nodules—bilobar distribution and multicentric nodules are also related to more aggressive tumor), many others factors have been related to recurrence, especially serum tumors biomarkers, histopathological features (microvascular invasion and poorly-differentiated tumors) and markers of inflammatory response (neutrophil-to-lymphocyte ratio and C-reactive protein (54). The latter has not achieved clinical use so far though and the routine use of nodule biopsy for patient selection is still a matter of debate, albeit being advocated by some centers (48). The main novel approach in the last years therefore has been the inclusion of serum biologic markers in selection criteria, since they can act as predictor of dropout or recurrence in patients with HCC. The most used ones are alpha-fetoprotein (AFP) and des-γ-carboxy prothrombin (DCP) (55). The initial serum levels and its variation during locoregional treatment are independent predictors of prognosis (56,57). They can be combined with traditional variables of tumors recurrence to select patients with more advanced HCC but favorable biological behavior, resulting in comparable outcomes to patients within MC.
Another recent identified independent prognostic factor is preoperative 18F-FDG PET uptake. Lee et al. (58) evaluated retrospectively 59 HCC patients who underwent LDLT according to PET positivity (defined as TSUVmax/LSUVmax of 1.15 or more). The 2-year recurrence-free survival rate was 97% for patients PET-negative and 42% for patients PET-positive (P<0.001). The same institution reported 22 patients with far advanced HCC (including 11 cases with macrovascular invasion) who underwent LDLT, the 2-year disease free survival rate was 23.9%. Nonetheless, the selected patients with low AFP (<200 ng/mL), and PET-negative, exhibited an outstanding 2-year recurrence-free survival of 66.7% (59).
On the other hand, some concern has been raised regarding the LDLT for HCC, when some series reported higher tumor recurrence in LDLT patients when compared to DDLT. In 2007, Fisher et al. (60) reported the initial results of the Adult-to-adult Living Donor transplantation Cohort Study (A2ALL), encompassing nine American centers. Fifty-eight LDLT were performed and the 3-years recurrence rate in LDLT and DDLT recipients was 29% and 0%, respectively (P=0.002). In 2012, Kulik et al. (40) published the updated results of A2ALL cohort, with 100 LDLT and 97 DDLT. The 5-year recurrence rate remained higher in LDL group (38% vs. 11%, P=0.01). In both reports, nevertheless, patients were more likely to have higher AFP serum levels, microvascular invasion, larger tumors or tumors beyond Milan and UCSF criteria. They also were less likely to receive pretransplant ablation therapy (59% vs. 79%, P=0.03), which reflected in a shorter transplant waiting time. Thereby, the authors concluded that the differences in outcome between LTLD and DDLT were more probably the result of patient selection rather than the type of graft per se. Despite the mentioned selection-bias, several hypothesis were made to explain these findings, including faster tumor progression due cytokine and growth factors released during liver regeneration, which is accentuated in LDLT (61,62). Another theory linked small-for-size grafts with higher endothelial growth-factor expression and consequently angiogenesis (63). More recent studies, however, have questioned these hypotheses (64).
The waitlist for transplantation seems to jeopardize recipients with potential curable HCCs, due the constant risk of cancer progression beyond accepted staging criteria. A recent meta-analysis reported dropout rates ranging from 9.2% to 31% (65). As the procedure is virtually done without any delay, LDLT minimizes this risk. Bhangui et al. (66) reported the waiting time for LDLT patients (2.8±2.4 months) significantly shorter than DDLT (7.9±9 months; P<0.001) in the same institution. Waiting time can be even shorter in others centers where LDLT is performed within a median of 44 days (25). Therefore, LDLT for patients with HCC is a more inclusive option, minimizing (or almost excluding) the risk of dropout.
Conversely, time can be tricky in LT for patients with HCC, since longer waiting time between being listed to transplant may offer better long-term outcomes. Patients with HCC waiting more than 120 days for DDLT have 1-year posttransplant recurrence rate significantly lower than patients waiting less than 120 days (2.2% vs. 3.9%, P=0.002) (67). Halazun et al. (68) evaluated the United Network for Organ Sharing (UNOS) data regarding DDLT recipients with HCC who had longer waiting time (median 7.6 months) with shorter waiting time (1.6 months). Longer waiting time was identified as a risk factor for death on the list (8.4% vs. 1.6%, P<0.001), while both intent-to-treat and post-transplant survivals were better in this group. A short waiting time was an independent predictor of poor patient survival on multivariate analysis. These paradoxical findings highlight time as important selection criteria. Waiting longer for LT could reveal the biological behavior of the tumor and then patients with aggressive HCC patterns would dropout before transplantation. Lai et al. (69) compared HCC recurrence after LDLT (n=116) or LT (n=157), interestingly in two different centers in Asia and Europe, respectively. They identified waiting time higher than 3 months before transplantation as a better outcome factor regardless the type of transplantation. Moreover, excluding salvage transplantations, recurrence rates were similar. Additionally, during longer waiting periods patients are usually treated with sequential locoregional therapy, such as radiofrequency ablation (RFA) and transarterial chemoembolization (TACE). Allard et al. (70) demonstrated that complete or nearly complete pathologic response after TACE improves long-term survival after LT independently of other pathological factors. Five-year overall and disease free survival after LT were higher in patients with complete pathological response compared to those without, 84% vs. 65% (P=0.09) and 94% vs. 73% (P=0.007), respectively. Finally, the “fast-track” effect commonly adopted in LDLT could lead to inferior long term outcomes (40).
An important ITT study was published by Azoulay et al. (41) in 2016 with five centers in France performing 79 LTLD and 782 DDLT. A total of 162 patients were removed from the list while waiting for DDLT, resulting in a drop-out rate of 20.7%. The major cause for removal was tumor progression (91.3%). The only contraindication for transplantation was extrahepatic disease or vascular invasion on radiological exams, irrespective of the level of involvement (from the main trunk to the segmental level). Interestingly, patients in LDLT group exhibited higher MELD and Child scores (14.7 vs. 12.7, P<0.01 and 35.9% of Child C vs. 23.4%, P=0.05), had larger tumors (2.97 vs. 2.67 cm, P=0.02) and had higher AFP serum levels (305 vs. 105 ng/mL, P=0.01). The proportion of patients undergoing TACE/RFA as bridge-therapy was similar in both groups (68.4% vs. 64.4%, P=0.2). Retransplantation and postoperative mortality were also similar (5.1% vs. 5.3%, P=0.92 and 7.6% vs. 7.7%, P=0.96, respectively). The ITT 5-year OS trended in favor of LDLT (73.2% vs. 66.7%, P=0.062). Multivariate analysis however identified LDLT and the time-of- listing MELD lower than 25 as predictors of better survival. It is noteworthy that this difference in survival no longer exists when comparing only transplanted patients (5-year OS: 73.2% vs. 73%, P=0.4). Regarding recurrence, the rates were similar between LDLT and DDLT (10.9% vs. 11.2%, P=0.8), nonetheless time to recurrence was longer in LDLT group (67.5 vs. 50.6 months, P<0.01). Multivariate analysis identified macrovascular invasion, tumors outside MC and AFP model score higher than 2 as independent predictors of recurrence. Even though multicentric, the strength of this series is that all patients were selected according to the same criteria and under the same allocation rules and transplanted by similar centers offering both modalities, during a limited span of time. Authors concluded that LDLT shortens waiting time and improves ITT OS, not representing a risk factor for recurrence. Thereby, it should be encouraged when available.
Liver grafts from living donors are typically of good quality, with minimal fat and reduced ischemic time. On the other side, LDLT is more complex than DDLT due surgical technical aspects and ethical issues that do not exist in DDLT arise: is it correct to risk the donor’s integrity, a healthy and active person, in favor of a cirrhotic patient with limited life expectative? Firstly, the donor safety must be the main priority. The donor procedure indeed presents 10% morbidity in high volume centers (71), but its incidence might be higher in low volume ones. Secondly, there is the ethical concern regarding exposing the donor to this risk when the recipient has advanced HCC with expected higher recurrence rate (72). For this reason, the institution should take a clear position and the transplantation team must provide full disclosure to donor and recipients. Finally, LDLT for patients with HCC beyond accepted criteria for deceased donation are usually forbidden to get access to re-transplantation in case of graft failure. Re-transplantation because of graft failure after LDLT is a rare event, although when needed, outcomes after LDLT are not different from DDLT (73). However, these patients would not have qualified for DDLT in the first place, then re-transplantation using a deceased donor is not recommended in this context (8).
In summary, Eastern countries have proved that LDLT has an outstanding potential for HCC patients. It allows the inclusion of patients with more advanced tumors without jeopardizing outcome, through a careful selection of patients with predictors of better prognosis, such as low values/favorable slopes of serological markers; PET negativity; response to locoregional therapies; and excluding patients with poor differentiation tumors. Timing to transplantation is also important, it might be managed to avoid too long or too short periods. Ideally, it should avoid excessive dropout, but must be long enough to identify tumors with aggressive biology. Western countries have a limited, but encouraging experience using LDLT for HCC patients. These countries should expand the indication of LDLT to patients with prolonged time in the waiting list in order to reduce dropout; and for patients presenting more advanced tumors, with predictors of favorable biological behavior.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Altekruse SF, McGlynn KA, Reichman ME. Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. J Clin Oncol 2009;27:1485-91. [Crossref] [PubMed]
- El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology 2007;132:2557-76. [Crossref] [PubMed]
- Njei B, Rotman Y, Ditah I, et al. Emerging trends in hepatocellular carcinoma incidence and mortality. Hepatology 2015;61:191-9. [Crossref] [PubMed]
- Sherman M. Hepatocellular carcinoma: epidemiology, surveillance, and diagnosis. Semin Liver Dis 2010;30:3-16. [Crossref] [PubMed]
- Alazawi W, Cunningham M, Dearden J, et al. Systematic review: outcome of compensated cirrhosis due to chronic hepatitis C infection. Aliment Pharmacol Ther 2010;32:344-55. [Crossref] [PubMed]
- Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med 1996;334:693-9. [Crossref] [PubMed]
- Bruix J, Sherman M, Llovet JM, et al. for the EASL panel of experts on HCC. Clinical management of hepatocellular carcinoma: conclusions of the Barcelona-2000 EASL conference. J Hepatol 2001;35:421-30. [Crossref] [PubMed]
- Clavien PA, Lesurtel M, Bossuyt PM, et al. Recommendations for liver transplantation for hepatocellular carcinoma: an international consensus conference report. Lancet Oncol 2012;13:e11-22. [Crossref] [PubMed]
- Llovet JM, Fuster J, Bruix J. Intention-to-treat analysis of surgical treatment for early hepatocellular carcinoma: resection versus transplantation. Hepatology 1999;30:1434-40. [Crossref] [PubMed]
- Raia S, Nery JR, Mies S. Liver transplantation from live donors. Lancet 1989;2:497. [Crossref] [PubMed]
- de Villa V, Lo CM. Liver transplantation for hepatocellular carcinoma in Asia. Oncologist 2007;12:1321-31. [Crossref] [PubMed]
- Hwang S, Lee SG, Joh JW, et al. Liver transplantation for adult patients with hepatocellular carcinoma in Korea: comparison between cadaveric donor and living donor liver transplantations. Liver Transpl 2005;11:1265-72. [Crossref] [PubMed]
- Karakayali H, Moray G, Sozen H, et al. Expanded criteria for liver transplantation in patients with hepatocellular carcinoma. Transplant Proc 2006;38:575-8. [Crossref] [PubMed]
- Lo CM, Fan ST, Liu CL, et al. Living donor versus deceased donor liver transplantation for early irresectable hepatocellular carcinoma. Br J Surg 2007;94:78-86. [Crossref] [PubMed]
- Allam N, Khalaf H, Fagih M, Al-Sebayel M. Liver transplant for hepatocellular carcinoma:experience in a Saudi population. Exp Clin Transplant 2008;6:14-24. [PubMed]
- Azzam AZ, Hegab B, Khalaf H, et al. Liver transplantation in patients with hepatocellular carcinoma: a single-center experience. Exp Clin Transplant 2011;9:323-8. [PubMed]
- Shirabe K, Taketomi A, Morita K, et al. Comparative evaluation of expanded criteria for patients with hepatocellular carcinoma beyond the Milan criteria undergoing living-related donor liver transplantation. Clin Transplant 2011;25:E491-8. [Crossref] [PubMed]
- Isik B, Ince V, Karabulut K, et al. Living donor liver transplantation for hepatocellular carcinoma. Transplant Proc 2012;44:1713-6. [Crossref] [PubMed]
- Kaido T, Ogawa K, Mori A, et al. Usefulness of the Kyoto criteria as expanded selection criteria for liver transplantation for hepatocellular carcinoma. Surgery 2013;154:1053-60. [Crossref] [PubMed]
- Park MS, Lee KW, Suh SW, et al. Living-donor liver transplantation associated with higher incidence of hepatocellular carcinoma recurrence than deceased-donor liver transplantation. Transplantation 2014;97:71-7. [Crossref] [PubMed]
- Akamatsu N, Sugawara Y, Kokudo N. Living-donor vs deceased-donor liver transplantation for patients with hepatocellular carcinoma. World J Hepatol 2014;6:626-31. [Crossref] [PubMed]
- Wan P, Zhang JJ, Li QG, et al. Living-donor or deceased-donor liver transplantation for hepatic carcinoma: a case-matched comparison. World J Gastroenterol 2014;20:4393-400. [Crossref] [PubMed]
- Xiao GQ, Song JL, Shen S, et al. Living donor liver transplantation does not increase tumor recurrence of hepatocellular carcinoma compared to deceased donor transplantation. World J Gastroenterol 2014;20:10953-9. [Crossref] [PubMed]
- Kim JM, Kwon CH, Joh JW, P, et al. Expanded criteria for liver transplantation in patients with hepatocellular carcinoma. Transplant Proc 2014;46:726-9. [Crossref] [PubMed]
- Ninomiya M, Shirabe K, Facciuto ME, et al. Comparative study of living and deceased donor liver transplantation as a treatment for hepatocellular carcinoma. J Am Coll Surg 2015;220:297-304.e3. [Crossref] [PubMed]
- Gunay Y, Guler N, Yaprak O, et al. Living Donor Liver Transplantation Outcomes for Hepatocellular Carcinoma Beyond Milan or UCSF Criteria. Indian J Surg 2015;77:950-6. [Crossref] [PubMed]
- Chen LP, Li C, Wen TF, et al. Can living donor liver transplantation offer similar outcomes to deceased donor liver transplantation using expanded selection criteria for hepatocellular carcinoma? Pak J Med Sci 2015;31:763-9. [PubMed]
- Hu Z, Qian Z, Wu J, et al. Clinical outcomes and risk factors of hepatocellular carcinoma treated by liver transplantation: A multi-centre comparison of living donor and deceased donor transplantation. Clin Res Hepatol Gastroenterol 2016;40:315-26. [Crossref] [PubMed]
- Togashi J, Akamastu N, Kokudo N. Living donor liver transplantation for hepatocellular carcinoma at the University of Tokyo Hospital. Hepatobiliary Surg Nutr 2016;5:399-407. [Crossref] [PubMed]
- Hong SK, Lee KW, Kim HS, et al. Living donor liver transplantation for hepatocellular carcinoma in Seoul National University. Hepatobiliary Surg Nutr 2016;5:453-60. [Crossref] [PubMed]
- Kim SH, Moon DB, Kim WJ, et al. Preoperative prognostic values of α-fetoprotein (AFP) and protein induced by vitamin K absence or antagonist-II (PIVKA-II) in patients with hepatocellular carcinoma for living donor liver transplantation. Hepatobiliary Surg Nutr 2016;5:461-9. [Crossref] [PubMed]
- Umeshita K, Inomata Y, Furukawa H, et al. Liver transplantation in Japan: Registry by the Japanese Liver Transplantation Society. Hepatol Res 2016;46:1171-86. [Crossref] [PubMed]
- Gondolesi GE, Roayaie S, Muñoz L, et al. Adult living donor liver transplantation for patients with hepatocellular carcinoma: extending UNOS priority criteria. Ann Surg 2004;239:142-9. [Crossref] [PubMed]
- Jonas S, Mittler J, Pascher A, et al. Living donor liver transplantation of the right lobe for hepatocellular carcinoma in cirrhosis in a European center. Liver Transpl 2007;13:896-903. [Crossref] [PubMed]
- Sotiropoulos GC, Lang H, Nadalin S, et al. Liver transplantation for hepatocellular carcinoma: University Hospital Essen experience and metaanalysis of prognostic factors. J Am Coll Surg 2007;205:661-75. [Crossref] [PubMed]
- Di Sandro S, Slim AO, Giacomoni A, et al. Living donor liver transplantation for hepatocellular carcinoma: long-term results compared with deceased donor liver transplantation. Transplant Proc 2009;41:1283-5. [Crossref] [PubMed]
- Vakili K, Pomposelli JJ, Cheah YL, et al. Living donor liver transplantation for hepatocellular carcinoma: Increased recurrence but improved survival. Liver Transpl 2009;15:1861-6. [Crossref] [PubMed]
- Bhangui P, Vibert E, Majno P, et al. Intention-to-treat analysis of liver transplantation for hepatocellular carcinoma: living versus deceased donor transplantation. Hepatology 2011;53:1570-9. [Crossref] [PubMed]
- Sandhu L, Sandroussi C, Guba M, et al. Living donor liver transplantation versus deceased donor liver transplantation for hepatocellular carcinoma: comparable survival and recurrence. Liver Transpl 2012;18:315-22. [Crossref] [PubMed]
- Kulik LM, Fisher RA, Rodrigo DR, et al. Outcomes of living and deceased donor liver transplant recipients with hepatocellular carcinoma: results of the A2ALL cohort. Am J Transplant 2012;12:2997-3007. [Crossref] [PubMed]
- Azoulay D, Audureau E, Bhangui P, et al. Living or Brain-dead Donor Liver Transplantation for Hepatocellular Carcinoma: A Multicenter, Western, Intent-to-treat Cohort Study. Ann Surg 2016. [Epub ahead of print]. [Crossref] [PubMed]
- Yao FY, Xiao L, Bass NM, et al. Liver transplantation for hepatocellular carcinoma: validation of the UCSF-expanded criteria based on preoperative imaging. Am J Transplant 2007;7:2587-96. [Crossref] [PubMed]
- Herrero JI, Sangro B, Pardo F, et al. Liver transplantation in patients with hepatocellular carcinoma across Milan criteria. Liver Transpl 2008;14:272-8. [Crossref] [PubMed]
- Silva M, Moya A, Berenguer M, et al. Expanded criteria for liver transplantation in patients with cirrhosis and hepatocellular carcinoma. Liver Transpl 2008;14:1449-60. [Crossref] [PubMed]
- Zheng SS, Xu X, Wu J, et al. Liver transplantation for hepatocellular carcinoma: Hangzhou experiences. Transplantation 2008;85:1726-32. [Crossref] [PubMed]
- Toso C, Trotter J, Wei A, et al. Total tumor volume predicts risk of recurrence following liver transplantation in patients with hepatocellular carcinoma. Liver Transpl 2008;14:1107-15. [Crossref] [PubMed]
- Mazzaferro V, Llovet JM, Miceli R, et al. Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: a retrospective, exploratory analysis. Lancet Oncol 2009;10:35-43. [Crossref] [PubMed]
- DuBay D, Sandroussi C, Sandhu L, et al. Liver transplantation for advanced hepatocellular carcinoma using poor tumor differentiation on biopsy as an exclusion criterion. Ann Surg 2011;253:166-72. [Crossref] [PubMed]
- Sugawara Y, Tamura S, Makuuchi M. Living donor liver transplantation for hepatocellular carcinoma: Tokyo University series. Dig Dis 2007;25:310-2. [Crossref] [PubMed]
- Lee SG, Hwang S, Moon DB, et al. Expanded indication criteria of living donor liver transplantation for hepatocellular carcinoma at one large-volume center. Liver Transpl 2008;14:935-45. [Crossref] [PubMed]
- Andraus W, Canedo BF, D'Alburquerque LA. Living donor liver transplantation in Brazil-current state. Hepatobiliary Surg Nutr 2016;5:176-82. [PubMed]
- Mazzaferro V, Bhoori S, Sposito C, et al. Milan criteria in liver transplantation for hepatocellular carcinoma: an evidence-based analysis of 15 years of experience. Liver Transpl 2011;17 Suppl 2:S44-57. [Crossref] [PubMed]
- Paranaguá-Vezozzo DC, Ono SK, Alvarado-Mora MV, et al. Epidemiology of HCC in Brazil: incidence and risk factors in a ten-year cohort. Ann Hepatol 2014;13:386-93. [PubMed]
- Lee HW, Suh KS. Expansion of the criteria for living donor liver transplantation for hepatocellular carcinoma. Curr Opin Organ Transplant 2016;21:231-7. [Crossref] [PubMed]
- Lai Q, Melandro F, Pinheiro RS, et al. Alpha-Fetoprotein and Novel Tumor Biomarkers as Predictors of Hepatocellular Carcinoma Recurrence after Surgery: A Brilliant Star Raises Again. Int J Hepatol 2012;2012:893103.
- Lai Q, Inostroza M, Rico Juri JM, et al. Delta-slope of alpha-fetoprotein improves the ability to select liver transplant patients with hepatocellular cancer. HPB (Oxford) 2015;17:1085-95. [Crossref] [PubMed]
- Fujiki M, Takada Y, Ogura Y, et al. Significance of des-gamma-carboxy prothrombin in selection criteria for living donor liver transplantation for hepatocellular carcinoma. Am J Transplant 2009;9:2362-71. [Crossref] [PubMed]
- Lee JW, Paeng JC, Kang KW, et al. Prediction of tumor recurrence by 18F-FDG PET in liver transplantation for hepatocellular carcinoma. J Nucl Med 2009;50:682-7. [Crossref] [PubMed]
- Lee KW, Yi NJ, Suh KS. Further expanding the criteria for HCC in living donor liver transplantation: when not to transplant: SNUH experience. Transplantation 2014;97 Suppl 8:S20-3. [Crossref] [PubMed]
- Fisher RA, Kulik LM, Freise CE, et al. Hepatocellular carcinoma recurrence and death following living and deceased donor liver transplantation. Am J Transplant 2007;7:1601-8. [Crossref] [PubMed]
- Man K, Fan ST, Lo CM, et al. Graft injury in relation to graft size in right lobe live donor liver transplantation: a study of hepatic sinusoidal injury in correlation with portal hemodynamics and intragraft gene expression. Ann Surg 2003;237:256-64. [Crossref] [PubMed]
- Shi JH, Huitfeldt HS, Suo ZH, et al. Growth of hepatocellular carcinoma in the regenerating liver. Liver Transpl 2011;17:866-74. [Crossref] [PubMed]
- Yang ZF, Poon RT, Luo Y, et al. Up-regulation of vascular endothelial growth factor (VEGF) in small-for-size liver grafts enhances macrophage activities through VEGF receptor 2-dependent pathway. J Immunol 2004;173:2507-15. [Crossref] [PubMed]
- Hu Z, Zhong X, Zhou J, et al. Smaller grafts do not imply early recurrence in recipients transplanted for hepatocellular carcinoma: A Chinese experience. Sci Rep 2016;6:26487. [Crossref] [PubMed]
- Menahem B, Lubrano J, Duvoux C, et al. Liver transplantation versus liver resection for hepatocellular carcinoma in intention to treat: An attempt to perform an ideal meta-analysis. Liver Transpl 2017;23:836-44. [Crossref] [PubMed]
- Bhangui P, Vibert E, Majno P, et al. Intention-to-treat analysis of liver transplantation for hepatocellular carcinoma:living versus deceased donor transplantation. Hepatology 2011;53:1570-9. [Crossref] [PubMed]
- Samoylova ML, Dodge JL, Yao FY, et al. Time to transplantation as a predictor of hepatocellular carcinoma recurrence after liver transplantation. Liver Transpl 2014;20:937-44. [Crossref] [PubMed]
- Halazun KJ, Patzer RE, Rana AA, et al. Standing the test of time: outcomes of a decade of prioritizing patients with HCC: results of the UNOS natural geographic experiment. Hepatology 2014;60:1957-62. [Crossref] [PubMed]
- Lai Q, Avolio AW, Lerut J, et al. Recurrence of hepatocellular cancer after liver transplantation:the role of primary resection and salvage transplantation in East and West. J Hepatol 2012;57:974-9. [Crossref] [PubMed]
- Allard MA, Sebagh M, Ruiz A, et al. Does pathological response after transarterial chemoembolization for hepatocellular arcinoma in cirrhotic patients with cirrhosis predict outcome after liver resection or transplantation? J Hepatol 2015;63:83-92. [Crossref] [PubMed]
- Shin M, Song S, Kim JM, et al. Donor morbidity including biliary complications in living-donor liver transplantation: single-center analysis of 827 cases. Transplantation 2012;93:942-8. [Crossref] [PubMed]
- Cronin DC 2nd, Millis JM. Living donor liver transplantation: The ethics and the practice. Hepatology 2008;47:11-3. [Crossref] [PubMed]
- Bittermann T, Shaked A, Goldberg DS. When Living Donor Liver Allografts Fail:Exploring the Outcomes of Retransplantation Using Deceased Donors. Am J Transplant 2017;17:1097-102. [Crossref] [PubMed]
Cite this article as: Pinheiro RS, Waisberg DR, Nacif LS, Rocha-Santos V, Arantes RM, Ducatti L, Martino RB, Lai Q, Andraus W, D’Albuquerque LA. Living donor liver transplantation for hepatocellular cancer: an (almost) exclusive Eastern procedure? Transl Gastroenterol Hepatol 2017;2:68.


