Technical success and short-term results of surgical treatment of gastrointestinal stromal tumors: an experience of three centers
Introduction
Since the discovery of the KIT and PDGFRA genetic mutations (1), as well as administration of tyrosine kinase inhibitors (2), our concept of molecular and clinical characteristics of gastrointestinal stromal tumors (GIST) has been considerably widened, which led to rapid and substantial changes in the approaches to the diagnostics and treatment of these tumors (3,4).
However, GISTs still represent a complex problem for surgical oncology. Although the methods of abdominal cancer treatment are presently well known and validated (5), there are no clear surgical algorithms for GISTs yet. International guidelines such as ESMO or NCCN provide a wide variety of possible treatment modalities; however, it lacks definite selection criteria (3,4). Currently, there is no unified concept of surgical interventions for GISTs located in different regions of gastrointestinal tract.
Due to characteristics of biological behavior of these tumors, which include a low rate of lymphatic metastasis and infiltrating growth, currently adopted practice such as economic resection of organs allows to perform procedure without lymph nodes dissection (6). As a result, it prompted surgeons to actively implement minimally invasive interventions in treatment of these tumors, which contributes to better functional outcomes of surgery without affecting overall and disease-free survival (OS/DFS). Currently, endoscopic submucosal dissection (ESD), submucosal tunnel endoscopic resections (STER), endoscopic full thickness resections (EFTR) and laparoscopic wedge resections are the most commonly used procedures. In addition, hybrid technologies such as rendezvous are used when endo- and laparoscopic visualizations are performed simultaneously (7,8).
Some eminent representatives of the Eastern surgical school are convinced that the practice of minimally invasive procedures should be expanded to large GISTs (9). At the same time, the European society of medical oncologists restricts laparoscopic surgery (LS) interventions only to small GISTs by arguing that this approach substantially increases the risks of damaging the fragile pseudocapsule that significantly worsens the prognosis (4). It should be also noted, that very few specialists currently use endoscopic resections due to their technical complexity. However, there is general increase in the interest for minimally invasive methods, i.e., endoscopic and laparoscopic techniques, as they allow a safe and reliable tumor resection and have a number of considerable advantages over traditional approach such as less severe postoperative pain, early realimentation, lesser risk of wound infection, reduced bleeding, and shorter hospital stay (10).
Objective
The main goal was to assess short-term outcomes of different surgical approaches for GIST and to describe certain technical parameters influencing the choice of a specific intervention.
Methods
We have performed a retrospective analysis from a prospectively documented database of 116 patients with localized forms of GIST who underwent surgical treatment between 2010 and 2016 at Federal State-Funded Budgetary Public Health Facility L.G. Sokolov’ Hospital N 122 of the Federal Medical and Biological Agency, Federal State-Funded Budgetary Facility N.N.Petrov’ Research Institute of Oncology Ministry of Health of the Russian Federation, and Federal State-Funded Budgetary Facility Saint-Petersburg Multifield Center Ministry of Health of the Russian Federation. The diagnosis was verified by immunohistochemistry in all patients. Written informed consent and Institutional Review Board approval were obtained before review of any patient material.
Eighty eight (75.8%) patients were admitted to hospitals according to scheduled hospitalization. The analysis of clinical presentation in these cases reveals that tumor was occasionally found in 67 patients (57.7%) during routine endoscopic examination. Twenty one patients (18.1%) sought medical attention due to abdominal bloating, epigastric discomfort, symptoms of disruption of abdominal food transit, a palpable lump in the abdomen, fatigue, weight loss, pain and others. All routinely admitted patients were fully examined including endoscopic ultrasonography and three-phase contrast-enhanced computer tomography of the chest and abdominal cavities. If it was necessary, medical diagnosis was done collectively in form of multidisciplinary team by surgeon, endoscopist, radiologist, oncologist, and pathologist. None of patients received neoadjuvant imatinib therapy.
The rest 28 (24.2%) patients were admitted due to life-threatening evidence of GISTs (gastrointestinal bleeding in 14.7%, perforation in 5.3%, and bowel obstruction in 4.1% cases). These patients underwent an emergency surgical intervention. Therefore, this group was not examined at all and only surgeons that were on duty made the decision.
The following was obtained without comparative analysis due to significant difference between each group of patients: baseline and clinicopathological data including patient demographics, clinical presentation of tumors, tumor size and location, characteristics of operation procedures (duration, intraoperative blood loss, frequency of R0-resection and fragmentation of tumor), postoperative complications and length of hospital stay. GIST pathology was defined according to the GIST Risk Calculator tool based on research from Dr. Heikki Joensuu (4).
Statistical analyses were performed using SPSS for Windows, version 19.0 (SPSS Inc., Chicago, IL, USA). Univariate analyses were performed using Student’s t-tests and Mann-Whitney U tests. A P value <0.05 was considered significant.
Results
There were 71 (61.2%) females and 45 (38.8%) males in the study. The average age of patients was 61.2±2.13 y.o. (under 50 y.o.: 17.0%; 50 to 70 y.o.: 58,5%; over 70 y.o.: 24.5%). There were no statistically significant differences in groups of different types of surgery with respect to demographics. The tumors were located in the stomach in 87 patients (75%), in the small intestine in 26 patients (22.4%), and extragastrointestinal GISTs were in 3 patients (2.6%). Notably, this is consistent with reference literature data (11,12).
The average size of resected specimen was 5.7±0.85 cm. According to approved TNM classification of GIST with T criterion based on tumor size (13), 22 (19.0%) patients were diagnosed with T1, 51 (44.0%) with T2, 27 (23.2%) with T3, and 16 (13.8%) with T4 tumors. There were no cases of regional lymph nodes involvement. In accordance with tumor morphology and intraoperative data the risk of recurrence was very low in 12 (10.3%), low in 25 (21.6%), intermediate in 46 (39.7%), and high in 33 (28.4%) cases.
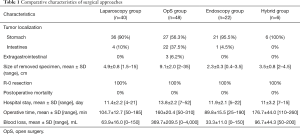
Forty (34.5%) patients underwent laparoscopic tumor resection. A traditional surgical intervention was performed in 48 (41.4%) patients; endoscopic procedures (EP) were performed in 22 (19.0%) patients, and a hybrid technique was used in 6 (5.1%) cases. Table 1 describes these surgical approaches. The most frequent procedures in open surgery (OpS) group were partial gastrectomy [13 (27%) patients] and small bowel resections [15 (31.3%) patients]. Moreover, in certain cases more extensive interventions were performed due to large size of the tumor or invasive growth with spread to nearby organs: 6 (12.5%) patients underwent multi-visceral excision of stomach, spleen and pancreas, 4 (8.3%) patients underwent subtotal gastrectomy, 2 (4.2%) patients underwent total gastrectomy, 1 (2.1%) patient underwent pancreatoduodenal resection, and 3 (6.3%) patients underwent excision of retroperitoneal lesion. Simultaneous interventions (tumor resection + cholecystectomy or sigmoid resection due to the presence of concomitant pathology) were performed in 4 (8.3%) patients. Of the 91 patients who underwent laparoscopic resections, 28 (70%) had wedge resection, 4 (10%) underwent subtotal gastrectomy, 3 (7.5%) underwent sleeve-resection, 2 (5%) underwent transgastric wedge-resection (in the cases of posterior gastric wall localization), 3 (7.5%) underwent small bowel resection. EPs included submucosal dissection in 6 (27.2%) patients, tunnel resections in 8 (36.4%) patients, and full-thickness resections in 8 (36.4%) patients.
Full table
All tumors were extracted without rupture. In 3 (7.5%) cases laparoscopic procedure was converted to laparotomy. In two patients it was due to the invasion of the tumor into the spleen and pancreas found during intraoperative revision. In another incident, tumor was located on posterior wall of the subcardial section of the stomach. In this case it was rather challenging to perform wedge resection because of high risk of postoperative stenosis of cardioesophageal junction.
All tumors were excised with negative microscopic margins (R0), and there was no postoperative mortality. Blood loss and surgical intervention duration were significantly higher in OpS compared to minimally invasive surgery group. At the same time, this may be explained due to the fact of traditional approach prevalence in patients with larger tumors (9.1 vs. 4.9 cm, P<0.05). In addition, patients who underwent OpS displayed a slight tendency to longer inpatient period; although, the difference is not statistically significant (13.1 vs. 11.5 days, P>0.05).
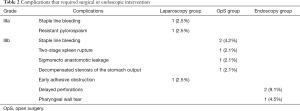
Overall complication rate and particular complications that required surgical or endoscopic intervention [grade IIIa and IIIb according to Clavien-Dindo classification (14)] were demonstrated in Table 2. It is clearly represented that early complications occurred slightly more frequently in endoscopic group than in OpS and laparoscopy (13.6% vs. 10.4% and 7.5%). There were no postoperative complications after hybrid “rendezvous” procedures.
Full table
Discussion
GISTs that originate from the intestinal cells of Cajal are rare but represent the largest subset of mesenchymal-derived tumor of the gastrointestinal tract (3-5). Every GIST is considered to have a potential to be malignant, and surgical removal is the only curative therapy for localized GISTs. Complete excision, avoiding tumor rupture is potentially curative and the mainstay of treatment for primary, resectable GIST (8,15,16).
Traditionally, GISTs resection was done with OpS, but more recently, less invasive methods has gained widespread acceptance. It is connected with several recent case series and systematic reviews which have reported that laparoscopic and endoscopic resections were superior to open resection in terms of short-term postoperative outcomes such as decreased blood loss, lower morbidity rate and shorter hospital stay without compromising the oncologic outcomes (7,12).
It is worth noting that it is technically possible to remove any tumor through laparoscopic or endoscopic approach, but it is imperative to remember that safe oncologic resection is the primary concern when considering minimally invasive techniques for GIST tumor rupture incomplete resections in these cases must always be avoided. Thus, each surgeon must proceed from his technical capabilities and maintain a delicate balance between the use of minimally invasive interventions and compliance with oncological requirements of surgery.
Despite the simplified surgical approach in stromal tumors in comparison with adenocarcinomas, the results of this study demonstrate a post-operative complications rate, 9.5% in overall study population. Since surgeons started practicing endoscopic and laparoscopic techniques in GIST surgery relatively recently, many surgeons still have to gain more experience. In addition, it could be relevant to the lack of interdisciplinary interaction between surgeons and endoscopists, wide application of staple technologies, absence of clear guidelines on surgery techniques, forced surgery in emergency hospital due to life-threatening signs of tumor. In order to solve this problem, it is essential to work out an optimal solution to pinpoint the most relevant surgical approach in regards of tumor localization, size, and growth pattern.
OpS is considered to be a method of choice for small intestine and extragastrointestinal tumors (2). Small bowel remains a difficult location for endoscopy and laparoscopy due to its lesser wall thickness in comparison to the stomach; and therefore, more complex manipulations are required. Nevertheless, there is a tendency to use laparoscopic approach, even in cases of tumors over 5 cm. Thus, in our study one patient with a tumor of 8 cm in diameter underwent a laparoscopic intestinal resection. Handport technique was used for a stromal tumor of approximately 15 cm in diameter in another case. Retroperitoneal tumors are seldom diagnosed at early stages and often reach considerable dimensions. Besides, the lack of clear anatomical marks creates further difficulty in application of laparoscopic technologies in these patients.
Thus, it is necessary to recognize that the stomach is the only field for the application of various minimally invasive technologies. Endoscopic approach is considered to be safe and feasible if tumor size that does not exceed 3 cm (17-19). This is determined by the high risk of complications during peroral extraction of larger tumor. We observed such incident in our study when posterior pharyngeal wall tear was registered after an attempt to remove 3.5 cm GIST. In addition, during an assessment stage by using endoscopic ultrasound, it is important to determine from which echo layer the lesion originates. If localization is in the muscularis mucosae, an ESD procedure is feasible because the perforation or bleeding risk is minimal (17,20). If the tumor originates from the muscularis propria, STER is preferable because this method prevents perforation due to maintaining of muscular layer continuity by forming a valve from mucosal and submucosal layers (18). If there is ulceration of overlying mucosa, fibrosis of submucosal layer resulting from previous biopsies or there is an intramural GIST attached by wide base, EFTR is the only possible endoscopic option. Thus, Zhou et al. (21) successfully removed tumors by EFTR in 26 patients with gastric GIST stemming from the muscularis propria. R0 resection was obtained in 100% of cases; the average tumor size was 2.8 cm (range 1.2 to 4.5 cm). Application of clip-assisted system OTSC together with twin grasper allowed effortlessly closing the wall defect of 3 cm or more (22,23). In our study, we observed two delayed perforations after applying this technique. Thereby, we came to conclusion that in some cases it is safer to perform laparoscopic closure of artificial perforations after EFTR.
It is evident that laparoscopic approach is safe and effective in GISTs under 5 cm (9,12,16). In our study, we demonstrated that laparoscopic approach could be applied in larger-sized tumors as well. Thus, 15 patients who had GISTs over 5 cm in size were successfully removed: 4 patients presented with 8 cm tumor, 4 with 7 cm and 7 with 6 cm in diameter. The low rate (7.5%) of conversion to OpS also highlights the feasibility of the laparoscopic approach. It is important to note, that conversion to OpS did not result in increased morbidity or mortality. Italian investigators revealed similar short-term results in retrospective case-control study of open versus laparoscopic resection of large GIST (24). Nevertheless, in the course of our study, patients underwent selection for a specific intervention, so there was bias in decision-making. Difficult-to-access GISTs with invasive growth were resected via OpS.
Surgery techniques can vary greatly depending on tumor localization and its relation to nearby structures. There is no need to perform gastric mobilization in extraluminal and transmural tumor growth on the anterior body wall and greater gastric curvature. The tumor is clearly visualized, patency and leakage are highly visible, and a stapled resection within healthy tissues can be monitored by a gauging probe with later endoscopic control of suture-line hemostasis. If the tumor is localized on the lesser gastric curvature, it is necessary to perform dissection on lesser gastric curvature with its partial mobilization before resecting the tumor in order to preserve the branches of vagus nerve leading to the antrum and gall bladder, which are needed to prevent post-operative complications. If the tumor is located close to cardioesophageal junction or pylorus, it is more advisable to perform gastric resection with unipolar coagulation or ultrasound scalpel prior to staple resection in order to remove as less surrounding healthy tissue as possible. This approach will have smaller risk of deformation and stenosis in the post-operative period (5). The most difficult localization for laparoscopic approach is a posterior wall of the stomach. Among our patients, this type of tumor location was found in three people. It is difficult to provide recommendations based on limited experience. However, it is worth mentioning that performing a gastrotomy with the following dislocation of tumor onto the anterior wall facilitates the operative procedure and does not require extensive mobilization of the stomach.
If a GIST under 5 cm in diameter with intraluminal or transmural growth type is located near cardioesophageal junction and pylorus and on the posterior wall of lesser curvature, it seems reasonable to perform hybrid interventions with simultaneous application of laparoscopic and endoscopic visualization in order to establish the exact borders of tumor and perform a precise resection and a reliable restoration of continuity of the gastric wall (25). Currently, we have gained experience of applying rendezvous technology in only 6 patients, but we hope to gain more experience in the future.
Finally, it should be mentioned that endoscopic and laparoscopic techniques are currently actively implemented in clinical practice for treatment of patients with GISTs (8). Minimally invasive procedures, as well as OpS, allow performing radical tumor resection. However, we are still experiencing a high rate of post-operative complications, which means that the problem of GIST surgical treatment still exists. Application of heterogeneous and non-standardized approaches represents a potential risk. The development of optimal solution based on tumor localization and topographic anatomy will allow us to improve safety and reliability of the treatment. Further analysis and more active implementation of modern approaches such as hybrid rendezvous (HR) techniques are required in order to increase effectiveness of localized GIST surgical treatments.
Our study is limited exclusively to a review of surgical tactics and early outcomes without analyzing of long-term results such as estimation of recurrence and survival rates as well as adjuvant imatinib administration. Also, there was relatively small sample size and the retrospective design. To further improve the management of GISTs, randomized double-blinded controlled trials have to be performed in order to compare the OpS versus laparoscopy and endoscopy for the treatment of these tumors.
Conclusions
There is a diversity of surgical modalities for GISTs treatment. From our point of view the main selection criteria for certain procedure are size, localization, growth type of the tumor and status of overlying mucosa. Nevertheless, due to relative rarity and heterogeneity of this pathology, individualization is necessary in each specific case. Laparoscopic and endoscopic surgery proved to be safe and feasible for resection of the gastric GISTs, with a reasonable operation time, low bleeding, and an acceptable complication rate. Moreover, immediate results indicate that all interventions were performed radically without mortality or serious morbidity.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: Written informed consent and Institutional Review Board approval were obtained before review of any patient material.
References
- Hirota S, Isozaki K, Moriyama Y, et al. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science 1998;279:577-80. [Crossref] [PubMed]
- Dagher R, Cohen M, Williams G, et al. Approval summary: imatinib mesylate in the treatment of metastatic and/or unresectable malignant gastrointestinal stromal tumors. Clin Cancer Res 2002;8:3034-8. [PubMed]
- Demetri GD, von Mehren M, Antonescu CR, et al. NCCN Task Force report: update on the management of patients with gastrointestinal stromal tumors. J Natl Compr Canc Netw 2010;8 Suppl 2:S1-41; quiz S42-4.
- ESMO/European Sarcoma Network Working Group. Gastrointestinal stromal tumours: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2014;25 Suppl 3:iii21-6. [Crossref] [PubMed]
- Chissov VI, Davydov MI. Oncology. National guideline. Moscow: GEOTAR-Media; 2014. Available online: http://www.rosmedlib.ru/book/ISBN9785970431535.html
- Sorour MA, Kassem MI. Gastrointestinal stromal tumors (GIST) related emergencies. Int J Surg 2014;12:269-80. [Crossref] [PubMed]
- Karachun AM, Kashchenko VA, Pelipas IV, et al. The current view on the features of surgical treatment of gastrointestinal stromal tumors. Clinical hospital 2016;16:48-57.
- Ko WJ, Cho JY. Current Techniques for Treating Gastrointestinal Stromal Tumors in the Upper Gastrointestinal Tract. Clin Endosc 2016;49:226-8. [Crossref] [PubMed]
- Lin J, Huang C, Zheng C, et al. Laparoscopic versus open gastric resection for larger than 5 cm primary gastric gastrointestinal stromal tumors (GIST): a size-matched comparison. Surg Endosc 2014;28:2577-83. [Crossref] [PubMed]
- De Vogelaere K, Hoorens A, Haentjens P, et al. Laparoscopic versus open resection of gastrointestinal stromal tumors of the stomach. Surg Endosc 2013;27:1546-54. [Crossref] [PubMed]
- Miettinen M, Lasota J. Gastrointestinal stromal tumors. Gastroenterol Clin North Am 2013;42:399-415. [Crossref] [PubMed]
- Valsangkar N, Sehdev A, Misra S, et al. Current management of gastrointestinal stromal tumors: Surgery, current biomarkers, mutations, and therapy. Surgery 2015;158:1149-64. [Crossref] [PubMed]
- Edge SB, Byrd DR, Compton CC, et al. Gastrointestinal stromal tumor. AJCC Cancer Staging Manual. 7th ed. New York: Springer, 2010.
- Clavien PA, Barkun J, de Oliveira ML, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg 2009;250:187-96. [Crossref] [PubMed]
- Miettinen M, Lasota J. Gastrointestinal stromal tumors: pathology and prognosis at different sites. Semin Diagn Pathol 2006;23:70-83. [Crossref] [PubMed]
- Nishida T, Hirota S, Yanagisawa A, et al. Clinical practice guidelines for gastrointestinal stromal tumor (GIST) in Japan: English version. Int J Clin Oncol 2008;13:416-30. [Crossref] [PubMed]
- Białek A, Wiechowska-Kozłowska A, Pertkiewicz J, et al. Endoscopic submucosal dissection for treatment of gastric subepithelial tumors (with video). Gastrointest Endosc 2012;75:276-86. [Crossref] [PubMed]
- Gong W, Xiong Y, Zhi F, et al. Preliminary experience of endoscopic submucosal tunnel dissection for upper gastrointestinal submucosal tumors. Endoscopy 2012;44:231-5. [Crossref] [PubMed]
- Lee IL, Lin PY, Tung SY, et al. Endoscopic submucosal dissection for the treatment of intraluminal gastric subepithelial tumors originating from the muscularis propria layer. Endoscopy 2006;38:1024-8. [Crossref] [PubMed]
- Hwang JC, Kim JH, Kim JH, et al. Endoscopic resection for the treatment of gastric subepithelial tumors originated from the muscularis propria layer. Hepatogastroenterology 2009;56:1281-6. [PubMed]
- Zhou PH, Yao LQ, Qin XY, et al. Endoscopic full-thickness resection without laparoscopic assistance for gastric submucosal tumors originated from the muscularis propria. Surg Endosc 2011;25:2926-31. [Crossref] [PubMed]
- Sarker S, Gutierrez JP, Council L, et al. Over-the-scope clip-assisted method for resection of full-thickness submucosal lesions of the gastrointestinal tract. Endoscopy 2014;46:758-61. [Crossref] [PubMed]
- Schmidt A, Bauerfeind P, Gubler C, et al. Endoscopic full-thickness resection in the colorectum with a novel over-the-scope device: first experience. Endoscopy 2015;47:719-25. [Crossref] [PubMed]
- Milone M, Elmore U, Musella M, et al. Safety and efficacy of laparoscopic wedge gastrectomy for large gastrointestinal stromal tumors. Eur J Surg Oncol 2017;43:796-800. [Crossref] [PubMed]
- Tsujimoto H, Yaguchi Y, Kumano I, et al. Successful gastric submucosal tumor resection using laparoscopic and endoscopic cooperative surgery. World J Surg 2012;36:327-30. [Crossref] [PubMed]
Cite this article as: Gluzman MI, Kashchenko VA, Karachun AM, Orlova RV, Nakatis IA, Pelipas IV, Vasiukova EL, Rykov IV, Petrova VV, Nepomniashchaia SL, Klimov AS. Technical success and short-term results of surgical treatment of gastrointestinal stromal tumors: an experience of three centers. Transl Gastroenterol Hepatol 2017;2:56.


