Minimally invasive surgery for gastric cancer in Brazil: current status and perspectives—a report from the Brazilian Laparoscopic Oncologic Gastrectomy Group (BLOGG)
Introduction
Since the first laparoscopic gastrectomy (LG) performed by Seigo Kitano (1) in Japan, in December 1991, this technique took several years until it was fully tested and adopted all over the world. In Brazil, to our knowledge, Dr. Renan Tinoco was the first to do this operation in Itaperuna, a city in the interior of Rio de Janeiro state (2).
After that pioneer there was a period of stagnation that progressively gave place to enthusiasm, especially when the digestive oncologic surgeons noticed that bariatric surgeons were doing advanced surgeries completely through minimally invasive approach.
In the years 2010 several Brazilian surgeons went to Korea and Japan to learn the technique and, since then, the minimally invasive gastrectomy is being performed in many Brazilian Hospitals, nevertheless there is still a long way until we will be able to do it in a regular basis.
Early years
A physician in Itaperuna, a small town of Rio de Janeiro State, made the first LG in Brazil in 1993. In a private hospital Dr. Renam C. Tinoco, a very skilled surgeon, begun to perform those cases and present his results in several Brazilian Congresses. The ability to perform obesity surgery could have contributed for him to inaugurate the LG in our country (2). Also in that hospital he could do it that because he had the payment for the equipment, while in the public that was possible only in a small number of cases with occasional support from the industry.
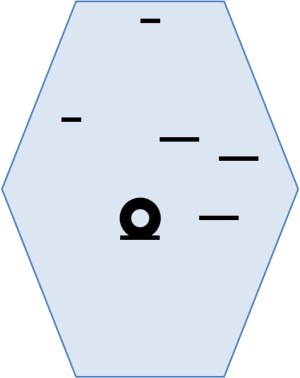
His first report appeared in a book chapter of Professors Eduardo Linhares, Laércio Lourenço and Takeshi Sano in 2005. At that time he used a different position for the trocars (Figure 1) and he located the tumors using a laparoscopic ultrasound probe (2).
Dr. Tinoco’s results were published in 2009, with 93 laparoscopic procedures, 77 for adenocarcinomas (44 subtotal and 33 total gastrectomies). In the subtotal gastrectomies, the reconstructions were Billroth II in 39 and Roux-en-Y in 5 cases, always with fully intracorporeal anastomosis. The technique used was a stapled side-to-side gastrojejunal anastomosis in the subtotal resections and a circular stapling in the total, with the anvil being orally introduced. There were 7 cases of conversion due to bleeding in three cases, pancreatic and splenic hilum invasion (2 cases), accidental colic vein ligation (colectomy was not needed) and the last case was due to intestinal adhesions. The morbidity rate was 14.1% and the mortality was 6.5% (5 cases) if we consider only the 77 cases of gastrectomy due to adenocarcinoma. The causes of death were: 2 esophagojejunal leaks and one pulmonary embolism in total gastrectomy cases, and two duodenal leaks in the subtotal cases. The number of harvested lymph nodes ranged from 21 to 57 and the mean surgical time was 162 minutes. There is no mention about long-term survival (3).
Between the years 2000 and 2010 we experienced a kind of stagnation regarding the gastrectomy due to cancer, on the other hand, there was an explosion in bariatric surgeries with almost all surgeries being totally performed through minimally invasive technique. The surgeons quickly gained skills in intracorporeal sutures. That phenomenon, associated to the news coming mainly from Asia, stimulated the ones involved in gastric cancer treatment to follow the same pathway, thus in the final of that decade and beginning of 2010 years, several surgeons went to Korea and Japan, in order to restart the gastrectomies in cancer patients and to obtain the necessary skills.
Years 2010–2015
In July 2011 the Institut de recherches contre le cancer de l’appareil digestif (IRCAD), institution dedicated to teach minimally invasive surgery, built its third unit in a city the interior of São Paulo state—Barretos. There they established a partnership with the Barretos Cancer Hospital (Pio XII Foundation). Under the leadership of Professor Jacques Marescaux from France and Professor Armando Melani from Brazil, and with the cooperation of Brazilian and foreign physicians, they begun to teach and train Brazilian and South American surgeons in minimally invasive gastrectomy. It was the definitive impulse necessary to spread the laparoscopic gastric cancer surgery.
In 2010 we had a big consensus meeting with colleagues from all of the country sponsored by the Brazilian Gastric Cancer Association (BGCA), in 2012 there was a confirmation of that consensus with the participation of several colleagues from abroad. Professors Maruyama, Kitagawa, Yang, De Manzoni, Roviello, Karpeh, Mansfield, Morgagni and others, helped us in that revision. In 2013 the BGCA published The Guidelines for Gastric Cancer in Brazil. One of the questions was: “Laparoscopic surgery can be used in the treatment of gastric cancer?” The answer was yes for 100% of the participating physicians (4).
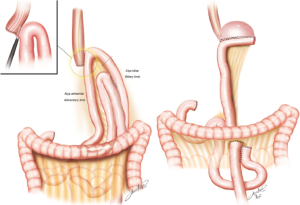
For the esophagojejunal anastomosis, Zilberstein (5) proposed and uses routinely, a side-to-side stapled anastomosis that seems simpler to be performed with the same success as the circular one. For the gastrojejunal in the subtotal cases an oralis totalis isoperistaltic reconstruction is used (Figure 2).
They operated 77 patients, 2 for GIST and 75 due to adenocarcinoma performing 55 subtotal and 22 total gastrectomies. The patients stayed in the hospital for 7 days in the subtotal and 13 in the total gastrectomies (mean). The stages in the adenocarcinoma cases were: stage IB—13 cases; stage IIA—27 cases; stage IIB—24 cases; stage IIIA—10 cases; stage IIIB—one case. There was only one morbidity (esophagojejunal leak) with spontaneous resolution after 50 days.
From the Barretos group, Lacerda, Bertulucci and Oliveira, published in 2014 their technique, including an interesting homemade reverse anvil method for the esophagojejunal circular anastomosis; in all cases the anastomosis was completely intracorporeal (6).
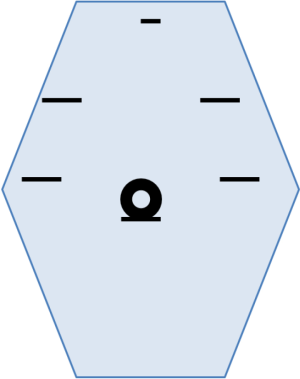
In 2015 they showed their experience comparing open (OTG) and laparoscopic total gastrectomy (LTG). In that paper they made a retrospective analysis comparing 64 open to 47 LTGs. The cases included advanced and early cancers. They already used a more traditional way for the trocars positioning, similar to those used by Korean and Japanese surgeons (7) (Figure 3).
The results showed shorter surgical time (216×255 minutes), earlier oral or enteral feeding and earlier hospital discharge. On the other hand there were a smaller number of harvested lymph nodes (28 in laparoscopic against 33 in open surgery), although in both groups they reached the goal to dissect more than 25 lymph nodes. There was no significant difference between the two groups regarding morbidity, mortality and reoperation rate.
With the participation of the Barretos/IRCAD group, which organized several courses during those years, the technique gained popularity and began to be performed in many centers in Brazil, especially in private hospitals because of lack of support of government to pay the staplers and the other materials necessary to that kind of operation1.
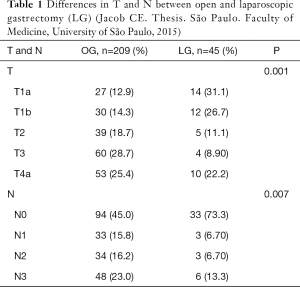
Reinforcing their strong will to introduce the gastrectomy in the academic entourage, the surgeons from the University of São Paulo, represented by Jacob2 published a thesis, analyzing the LG and comparing with the open procedure. In that study he analyzed 209 open gastrectomy (OG) and compared with 45 LG. It was included subtotal and total resections. The groups were comparable except the stages; the advanced cases were more frequent in the OG group. When analyzed separately also the T and N parameters were statistically different with more early and non-metastatic cases in the LG group (Table 1).
Full table
The results showed shorter operative time in the LG, but no difference regarding transfusion, hospital stay (median of 14 days for OG against 12 in LG), morbidity (31% in OG vs. 24% in LG), or reoperations 6.6% in open vs. 14% in LG). The morbidity and mortality were also similar in both groups (Tables 2,3).
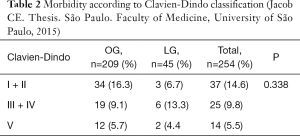
Full table
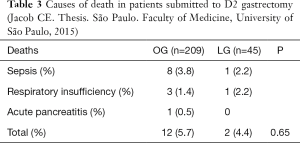
Full table
In this study the comparison between the groups obviously showed better survival in LG due to more patients in early stages, but it is important to emphasize that the 5-year survival in the LG reached 93.2%.
Aiming more efforts to obtain large number of patients submitted to minimally invasive gastrectomy, in 2016 we organized a multicentric group entitled the BLOGG (Brazilian Laparoscopic Oncologic Gastrectomy Group) including three institutions: A.C. Camargo Cancer Center, Santa Casa of São Paulo Medical School and the Faculty of Medicine of the University of São Paulo. All groups have large experience and tradition in treating gastric cancer and in each one, there were surgeons trained in Korea or Japan to perform LG.
In the first part we tried to collect data from those hospitals and for that, we created an automatic Excel sheet including the following data: age, gender, surgery date, extent of resection, reconstruction, anastomosis (assisted or totally laparoscopic), conversion rate, lymph node dissection, combined resections, morbidity, 30 day mortality, R status, histological type according to Laurén classification, margins, total and positive lymph nodes, stage, adjuvancy or neoadjuvancy, recurrence and follow up (alive or dead, with or without disease). One hundred and forty eight patients were operated from 2006 to 2016. There were 92 male and 56 female with ages ranging from 30 to 89 years (mean of 59.9 years and median of 61 years); there were 62 (41.9%) early, 79 (53.3%) advanced and 7 (4.7%) TX cases (complete pathological response after chemotherapy). Three cases were palliative. There were 98 subtotal, 48 total and 2 proximal gastrectomies. The anastomoses were totally laparoscopic in 105, laparoscopic assisted in 21, cervical in 2, and 20 open (after conversion). The reconstruction methods were: 142 Roux-en-Y, two Billroth I, and three other types. The conversion rate was 13.5% (20/148). The D2 dissection was performed in 139 patients. The mean number of lymph nodes was 34.4 (median of 31). If we take only the D2 cases the mean number was 39.5 (median of 36.5). The morbidity rate was 22.3%. Among the conversion patients the morbidity was 35% and in the exclusively laparoscopic was 20%. The mortality was 2.7% (4/148). The stages were: IA—59, IB—14, IIA—11, IIB—15, IIIA—9, IIIB—19, IIIC—11 and stage IV—three cases. Four patients died from the disease and ten are alive with disease.
The next step of the BLOGG is to begin a prospective study including early and advanced cases.
Current status and perspectives
Regarding the technique it seems that there are no more big obstacles to include the LG in the regular procedures. The trocar position adopted is the one proposed by most services around the world (Figure 3). The anastomoses in almost all services are fully intracorporeal, with circular or linear laparoscopic stapling devices. The participation of bariatric surgeons was important because they acquired the skills in intracorporeal sutures before the oncologic surgeons3.
Although the pioneers from Korea and Japan are still studying the role of LG in advanced gastric cancer, in our country the advanced cases are already being operated. In the services with higher number of cases the initial experience is being performed after approval of the scientific and ethical committees.
When we analyze the progress of the minimally invasive surgery in Brazil, we note that the economic problems have strong influence. The public system does not have enough resources to finance the equipment to perform laparoscopic surgery for most procedures. The strongest evidence of that is the cholecystectomy, which is still performed in the great part of the cases through a laparotomy1.
The public health services recognized and approved the laparoscopic method for most abdominal surgeries, but, incredibly, although approved, the government does not pay the public hospitals for that. The full payment (including housing, personnel, antibiotics and other medicaments) for a subtotal gastrectomy in a Hospital belonging to the public system is approximately U$500; for a total gastrectomy this amount is around U$1,500. On the other hand if we analyze only the prices of the dischargeable materials needed to perform those operations such as trocars, sealing and stapling devices, the prices rises up to U$5,000. This is due particularly to federal fees needed to import those products1.
It is well known in Brazil that the majority of cases of gastric cancer are operated in public hospitals. This condition has led to a situation where many surgeons perform the LG, but the cases in the private are rare and do not allow the surgeons to be well trained. On the other hand the public hospitals and universities are struggling to begin to perform the minimally invasive gastrectomy in a regular basis, but facing all kinds of difficulties regarding the price of the material. The other issue is that in the private practice the specific knowledge of the stomach cancer is sometimes belated and the advances in that field are not quickly incorporated to the daily practice.
Finally we have to mention the introduction of robotic procedures that are very similar to laparoscopic ones, give more precision and, we can say, more opportunity to enhance minimal invasive approach. The experience is very new, since robotic surgery was introduced in Brazil in the recent years, but it represents for sure a great advance in laparoscopic surgery for gastric cancer. We expect that, with wider access to robot training extended to a larger number of specialized surgeons and the popularization of the technique, this will become one of the new standards to gastrectomy.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
1 Kassab P, Malheiros CA. Freitas WR. Laparoscopic gastrectomy in Brazil. Personal communication. 7th Japan-Korea Laparoscopic Gastrectomy Joint Seminar. Beppu, Oita, 2012.
2 Jacob CE. Resultados da gastrectomia laparoscópica com linfadenectomia D2 no tratamento cirúrgico do câncer gástrico. (Thesis). São Paulo. Faculty of Medicine, University of São Paulo, 2015.
3 Malheiros CA, Kassab P, Freitas WR. Bariatric surgery in Brazil. Personal communication. 7th Japan-Korea Laparoscopic Gastrectomy Joint Seminar. Beppu, Oita, 2012.
References
- Kitano S, Iso Y, Moriyama M, et al. Laparoscopy-assisted Billroth I gastrectomy. Surg Laparosc Endosc 1994;4:146-8. [PubMed]
- Tinoco RC, Tinoco AC. A Laparoscopia no câncer gástrico. In: Eduardo LinharesE, Lourenço L, Sano T. editors. Atualização em Câncer Gástrico. 1ed. São Paulo, SP: Tecmedd Editora, 2005:243-9.
- Tinoco RC, Tinoco AC, El-Kadre LJ, et al. Laparoscopic gastrectomy for gastric cancer. Surg Laparosc Endosc Percutan Tech 2009;19:384-7. [Crossref] [PubMed]
- Zilberstein B, Malheiros C, Lourenço LG, et al. Brazilian consensus in gastric cancer: guidelines for gastric cancer in Brazil. Arq Bras Cir Dig 2013;26:2-6. [Crossref] [PubMed]
- Zilberstein B, Jacob CE, Barchi LC, et al. Simplified technique for reconstruction of the digestive tract after total and subtotal gastrectomy for gastric cancer. Arq Bras Cir Dig 2014;27:133-7. [Crossref] [PubMed]
- Ramagem CA, Linhares M, Lacerda CF, et al. Comparison of laparoscopic total gastrectomy and laparotomic total gastrectomy for gastric cancer. Arq Bras Cir Dig 2015;28:65-9. [Crossref] [PubMed]
- Lacerda CF, Bertulucci PA, Oliveira AT. Step-by-step esophagojejunal anastomosis after intra-corporeal total gastrectomy for laparoscopic gastric cancer treatment: technique of "reverse anvil". Arq Bras Cir Dig 2014;27:71-6. [Crossref] [PubMed]
Cite this article as: Kassab P, Costa WL Jr, Jacob CE, Cordts RM, Castro OA, Barchi LC, Cecconello I, Charruf AZ, Coimbra FJ, Cury AM, Diniz AL, Farias IC, Freitas WR Jr, Godoy AL, Ilias EJ, Malheiros CA, Ramos MF, Ribeiro HS, Roncon Dias A, Thuler FR, Yagi OK, Lourenço LG, Zilberstein B; on behalf of the Brazilian Gastric Cancer Association. Minimally invasive surgery for gastric cancer in Brazil: current status and perspectives—a report from the Brazilian Laparoscopic Oncologic Gastrectomy Group (BLOGG). Transl Gastroenterol Hepatol 2017;2:45.


