The evolving landscape of treatment for advanced gastric cancer and the role of anti-angiogenic therapy: implications from results of the INTEGRATE study
Gastric cancer (GC) is the fifth most common malignancy worldwide. Nearly 2/3 of all cases are in developing countries in Eastern Europe, South America, and Asia, 42% of all new cases are in China (1). Empiric chemotherapy has been the mainstay of treatment for patients presenting with advanced/metastatic disease, to help reduce symptoms and prolong survival. In the first line setting a fluoropyrimidine backbone is utilized (5-FU, capecitabine, S1), in combination with a platinum agent (cisplatin, oxaliplatin) or a taxane (2). Therapy has evolved in a stepwise fashion, with incremental gains with each additional agent to this backbone, with doublet then triplet chemotherapy leading to improvements in median overall survival (OS), but at the expense of greater toxicity. Randomised controlled trials have further confirmed the benefit of chemotherapy in the second line setting with paclitaxel, docetaxel and irinotecan (3,4). The next key evolution of therapy has been the addition of a biologic agent to chemotherapy and the use anti-angiogenic therapy in refractory disease.
The first successful phase III trial of a biologic agent in GC targeted the human epidermal growth factor receptor 2 (HER2) transmembrane signalling protein. The ToGA study (5) evaluated trastuzumab, a humanized monoclonal antibody to HER2 in combination with capecitabine and cisplatin. The benefit of adding trastuzumab was greatest in the highly HER2 overexpressing population, median OS, 16.1 vs. 11.8 months; P=0.0046, making this a new standard of care (5).
The next successful biologic targeted therapies in advanced GC have been those inhibiting or targeting tumour angiogenesis, important for primary tumour growth and metastasis formation. Tumour over-expression of vascular endothelial growth factor (VEGF) and high circulating VEGF levels in GC has been associated with a poorer prognosis. The AVAGAST study (6) was the first phase III study to test the addition of an antiangiogenic agent (bevacizumab, a monoclonal antibody to VEGF) to first-line chemotherapy in advanced GC. Although the study was negative for survival, there was some evidence of activity with the addition of bevacizumab improving progression free survival [PFS, 6.7 vs. 5.3 months; hazard ratio (HR) 0.80; P=0.0037] and response rate (RR) (6).
Ramucirumab, a fully human immunoglobulin (Ig) G1 monoclonal antibody targeting VEGF receptor (R)-2 was the next anti-angiogenic targeted therapy to show benefit, demonstrating improved survival both as monotherapy [in the REGARD study (7)] and in combination with paclitaxel [in the RAINBOW study (8)], both in the second-line setting. In the REGARD study, patients receiving ramucirumab had a significantly improved OS, HR 0.78 (95% CI, 0.603 to 0.998; P=0.047) compared with those receiving placebo (7). In the RAINBOW study, median survival with the addition of ramucirumab to paclitaxel was 9.6 months compared with 7.4 months for paclitaxel alone (HR 0.81; P=0.017). Furthermore, PFS was also improved (HR 0.635; median PFS: ramucirumab plus paclitaxel, 4.4 months vs. paclitaxel alone, 2.9 months; P<0.0001) (8).
The other key class of anti-angiogenic agent to be explored in advanced GC has been the small molecule tyrosine kinase inhibitors. Apatinib, an oral small-molecule inhibitor of VEGFR-2 was first to be examined in a phase III placebo controlled study in the third-line setting in China. The study demonstrated improved PFS with apatinib and improved OS (HR 0.71; 95% CI, 0.54 to 0.94; P=0.0017) (9).
Taken collectively, these studies confirm the angiogenic phenotype of GC and its susceptibility to anti-angiogenic therapy. Regorafenib (BAY 73-4506) is an oral multikinase inhibitor with a broad profile, targeting angiogenic (VEGF-R1, VEGF-R2, and TIE-2), stromal (platelet-derived growth factor receptor beta), and oncogenic (RAF, RET, and KIT) receptor tyrosine kinases, that had previously demonstrated efficacy in refractory gastrointestinal stromal tumor (GIST) (10) and colorectal cancer (11). INTEGRATE was an international (Australia and New Zealand, South Korea, and Canada) multicentre randomized placebo-controlled phase II trial, led by the Australasian Gastrointestinal Trials Group (AGITG) that was undertaken to determine whether regorafenib had sufficient activity and safety to warrant further evaluation in a phase III trial in refractory GC (12). It commenced in November, 2012, at a time before the results of the ramucirumab and apatinib studies were reported and completed accrual by February 2014.
Patients with refractory advanced GC or esophagogastric cancer, were randomly assigned in a two-to-one ratio, stratified by line of prior chemotherapy for advanced disease (one vs. two) and geographic region. Eligible patients received best supportive care plus regorafenib 160 mg or matching placebo orally on days 1 to 21 of each 28-day cycle until disease progression or prohibitive adverse events occurred. One hundred and forty seven patients (of 152 enrolled) were eligible for inclusion in the efficacy analysis. Baseline characteristics were balanced with the primary tumor location esophagogastric junction in 38% and stomach in 58% of patients. Exposure to prior chemotherapy was 42% (one line of therapy) and 58% (two lines of chemotherapy). Eastern Cooperative Oncology Group performance status was 0 (42%) or 1 (58%). HER2 status was known in 85 patients (56%), but only six patients received trastuzumab. The dominant site of metastasis was the liver (12).
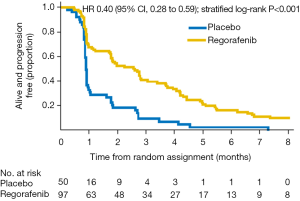
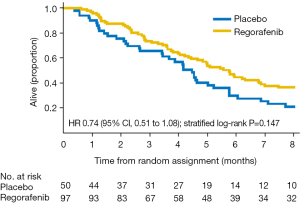
The median duration of active treatment was 1.8 months (regorafenib) and 0.9 months (placebo). The primary outcome of the study was PFS in the regorafenib arm. Responses were assessed locally using RECIST (v1.1) criteria. Blinded central review of all RECIST source documents was undertaken for all patients for date of progression, and 13% of patients were randomly selected for blinded central review by two independent radiologists. PFS significantly differed between the groups, from 2.6 months (regorafenib group) to 0.9 months (placebo group) (HR 0.40; P=0.001) (Figure 1) (12). This effect was greatest in patients from South Korea than in Australia, New Zealand, and Canada combined (HR 0.12 vs. 0.61; interaction P=0.001), but consistent across all other sub-groups (age, neutrophil-to-lymphocyte ratio, primary site, lines of therapy, presence of peritoneal metastases, number of sites of metastatic disease, and baseline plasma VEGF-A). Despite over half the patients in the placebo arm crossing over to receive open-label regorafenib, a survival trend was seen in favour of regorafenib (5.8 vs. 4.5 months; HR 0.74; P=0.147) (Figure 2) (12).
Several covariates were tested as prognostic factors. Higher baseline neutrophil-to-lymphocyte ratio was an independent prognostic factor for OS (HR 1.82; P=0.001) (12). Independent prognostic factors for PFS were age older than 60 years (HR 0.68; P=0.04) and neutrophil-to-lymphocyte ratio (HR 1.56; P=0.01) (12). More than two lines of therapy and number of metastatic sites were also prognostic factors for PFS in univariate analyses. After adjustment for significant prognostic factors, the treatment effect on PFS remained highly statistically significant (HR 0.39; P<0.001), and there was a trend toward improved survival (HR 0.70; P=0.06) (12).
In the INTEGRATE study, regorafenib was generally well tolerated, with similar toxicity as reported in the GIST and colorectal studies (10-12). There was no detriment to quality of life in patients receiving regorafenib compared with placebo (12). Final exploratory biomarker analyses on tissue and blood samples is underway, including investigation of VEGF-related biomarkers and other biomarkers relating to angiogenesis and/or tumorigenesis in blood and tumour including FGF pathway, PDGF, vWF, TIE-1 and -2.
The greater PFS benefit of regorafenib in the South Korean patient cohort was not explained by other factors in a multivariable analysis and contradicted the observation of a lower OS seen with bevacizumab in Asians in the AVAGAST study (6,12). Possible explanations include differences in genetic polymorphisms predicting for differential drug response, differentially distributed gastric molecular phenotypes across geographic populations conferring differing prognoses and drug sensitivity (13), pharmacokinetic differences by population, or a combination thereof.
Advances in molecular technology have led to a greater understanding of the molecular pathogenesis of GC. Recently, the Cancer Genome Atlas analysis demonstrated four key molecular subtypes of GC: chromosomal instability, mismatch repair, genomically stable and Epstein-Barr virus (EBV) subtypes of GC with each subtype unique, each having implications for drug development (14). Ideally, it would be important to know the distribution of these subtypes by geographic region and within future studies, to better understand their drug sensitivity. Opportunity exists to focus on these targets in addition to the ongoing trials with other anti-angiogenic agents and the new immune checkpoint inhibitors such as PD-1/PD-L1 inhibitors.
In summary, GC is a major global health issue. Chemotherapy remains the mainstay of therapy of metastatic disease. Several anti-angiogenic agents have demonstrated activity, with ramucirumab now registered for use in several countries. The international multicenter AGITG led Phase II INTEGRATE Study of regorafenib demonstrated promising activity in the refractory setting in advance GC, without detriment in patient quality of life. As such, an international (ANZ, Canada, USA, South Korea, Japan, and Taiwan) multicenter phase III trial of regorafenib (or placebo) in advanced refractory GC has opened to recruitment with OS as the primary endpoint (ACTRN12616000420448). If positive, it will establish a role for regorafenib as a treatment option in refractory advanced GC.
Acknowledgements
None.
Footnote
Conflicts of Interest: Nick Pavlakis is a consultant and has received honoraria from Roche, Eli Lily, Bayer; and has received grant funding from Bayer. Malinda Itchins has received honorarium from Pfizer, and has received travel support from Sanofi, Novartis, Amgen, BMS.
References
- Brenner H, Rothenbacher D, Arndt V. Epidemiology of stomach cancer. Methods Mol Biol 2009;472:467-77. [Crossref] [PubMed]
- Shah MA. Update on metastatic gastric and esophageal cancers. J Clin Oncol 2015;33:1760-9. [Crossref] [PubMed]
- Kang JH, Lee SI, Lim DH, et al. Salvage chemotherapy for pretreated gastric cancer: a randomized phase III trial comparing chemotherapy plus best supportive care with best supportive care alone. J Clin Oncol 2012;30:1513-8. [Crossref] [PubMed]
- Hironaka S, Ueda S, Yasui H, et al. Randomized, open-label, phase III study comparing irinotecan with paclitaxel in patients with advanced gastric cancer without severe peritoneal metastasis after failure of prior combination chemotherapy using fluoropyrimidine plus platinum: WJOG 4007 trial. J Clin Oncol 2013;31:4438-44. [Crossref] [PubMed]
- Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 2010;376:687-97. [Crossref] [PubMed]
- Ohtsu A, Shah MA, Van Cutsem E, et al. Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: a randomized, double-blind, placebo-controlled phase III study. J Clin Oncol 2011;29:3968-76. [Crossref] [PubMed]
- Fuchs CS, Tomasek J, Yong CJ, et al. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet 2014;383:31-9. [Crossref] [PubMed]
- Wilke H, Muro K, Van Cutsem E, et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol 2014;15:1224-35. [Crossref] [PubMed]
- Li J, Qin S, Xu J, et al. Randomized, Double-Blind, Placebo-Controlled Phase III Trial of Apatinib in Patients With Chemotherapy-Refractory Advanced or Metastatic Adenocarcinoma of the Stomach or Gastroesophageal Junction. J Clin Oncol 2016;34:1448-54. [Crossref] [PubMed]
- Demetri GD, Reichardt P, Kang YK, et al. Efficacy and safety of regorafenib for advanced gastrointestinal stromal tumours after failure of imatinib and sunitinib (GRID): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 2013;381:295-302. [Crossref] [PubMed]
- Grothey A, Van Cutsem E, Sobrero A, et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 2013;381:303-12. [Crossref] [PubMed]
- Pavlakis N, Sjoquist KM, Martin AJ, et al. Regorafenib for the Treatment of Advanced Gastric Cancer (INTEGRATE): A Multinational Placebo-Controlled Phase II Trial. J Clin Oncol 2016;34:2728-35. [Crossref] [PubMed]
- Miyamoto N, Yamamoto H, Taniguchi H, et al. Differential expression of angiogenesis-related genes in human gastric cancers with and those without high-frequency microsatellite instability. Cancer Lett 2007;254:42-53. [Crossref] [PubMed]
- Cancer Genome Atlas Research Network. Comprehensive molecular characterization of gastric adenocarcinoma. Nature 2014;513:202-9. [Crossref] [PubMed]
Cite this article as: Itchins M, Pavlakis N. The evolving landscape of treatment for advanced gastric cancer and the role of anti-angiogenic therapy: implications from results of the INTEGRATE study. Transl Gastroenterol Hepatol 2017;2:29.