Deciphering the pathophysiology of irritable bowel syndrome and functional gastrointestinal disorders—an alternative model for pathogenesis: cytokine controlled transepithelial multi-feedback loop
Introduction
A theoretical model of disease pathogenesis can provide useful touch points for investigation. Focusing analysis on touch points permit elucidation of the pathophysiology that defines the disease. Directed investigations assist in deciphering critical determinants that predict disease as oppose to observations that simply characterize features of the disorder without uncovering its true determinants.
Therefore, having a reliable model is indispensable in deciphering which alterations in physiology actually give rise to disease.
Irritable bowel syndrome (IBS) is a clinically defined disorder characterized by symptoms and signs that lend no clear entrance to its pathogenesis. The “brain-gut axis” approach, recently renamed the “gut-brain interaction” serve as an efficient collection of prominent features of the disease consisting of relevant cohorts of symptom/sign observations that characterize IBS and other functional gastrointestinal disorders (FGIDs) (1). The seminal traits of abnormalities in IBS are clinical (1,2), histological (3-5), histoimmunological (6-11) and neurophysiologic (12,13). Added to these are new observations related to the enteric microbiome (14) and its relationship to IBS; this gut-brain relationship between enteric microbiome and central nervous system can be visualized by physiologic neuro-imaging (15,16).
Instead of providing a working model, these observations present characteristics of IBS that are short of identifying causative determinants. It is likely that these observations represent the physiologic effects of yet to be determined cause or causes that induce the syndrome. This is true of the other functional GI disorders including non-erosive reflux, functional dyspepsia, and esophageal pain syndrome. Investigations have uncovered many features or traits, with no single one or two observations able to direct investigational efforts toward a definitive identification of the origin of these disorders.
An alternative starting point—effective therapeutic intervention
An alternative starting point for any pathogenesis model is an effective therapeutic intervention—an intervention that brings about rapid and complete reversal of disease, that is, complete amelioration of signs and symptoms that define the disorder. This occurred with the discovery of insulin, various classes of antibiotics and targeted immuno-therapies for psoriasis, rheumatoid arthritis and certain cancers. The introduction of an effective therapeutic intervention provides a cause-effect starting point around which a model of pathogenesis could be constructed, complete with touch points for further investigation and discovery. More often than not, advances in medicine and physiology come by way of models fashioned from a curative outcome of an effective intervention. Thus the therapeutic intervention served to focus research efforts at known mechanism of action specific to that intervention.
What constitute an effective therapeutic intervention?
Rapid and complete reversal of a disease process at a rate that is 10-fold greater than otherwise expected is termed a positive Glasziou treatment effect (17) qualifies as an effective therapeutic intervention. The difference in effect size between treatment and placebo is roughly 10–50 base points (18). Therefore comparisons between placebo and any prospective intervention require control of biases that confound outcomes. From his work at the Oxford Center of Evidence Based Medicine, Glasziou concluded that while randomized trials aptly and quantitatively discern a true treatment signal from noise of experimental bias, the magnitude of the treatment effect of certain interventions, can be so dramatic that experimental bias can be statistically ruled out as an explanation of the observed treatment effect. Glasziou (17) asserted that implausibly large associations, both between treatment and confounding factors and between confounding factors and outcome, are required to explain comparative response rates of 5–10. He concluded that rate ratios (obtained by comparing time to point of improvement between any prospective and standard intervention) that are beyond 10 reflect real treatment effects of the prospective intervention, even if confounding factors (experimental bias) were uncontrolled. In other words, the contributions of uncontrolled experimental bias would be trivial and non-determinative to the treatment outcome, having a P value of 0.05 or less.
Six weeks of specific clinical symptoms and signs over a six month period are required to confirm the diagnosis of IBS (constipative or diarrhea dominant). As reported elsewhere (19-21) persisting symptoms and signs of IBS can be eliminated 2–3 days following the use of high potency polymerized cross-linked sucralfate (HPPCLS). Compared to the time to treatment effect for other FDA approved agents for IBS—lubiprostone, linaclotide, rifaximin, alosetron and tegaserod—this 2–3 day elimination of symptoms and signs of IBS represents a rate ratio range from 14–56. This is well beyond the required multiple of 10 for a positive Glasziou treatment effect. While this repeated observation reported by our institution indeed requires reproduction by other investigators, the emergence of this outcome compels a proposal for an alternative model of IBS pathogenesis. A model stemming from the clinical outcome of HPPCLS is one based on syndrome reversal associated with an intra-luminal non-systemic, topically acting therapy having no obvious mucosal targets—a model based on the therapeutic efficacy of HPPCLS.
IBS model based on HPPCLS therapeutic efficacy
The model presented here accommodates the known mechanism of action inherent to the intervention and the known mechanisms of actions involved in mucosal inflammation (5-7). Once ingested, HPPCLS acts as a muco-adherent, topically acting non-systemic agent (22-24). Its entire therapeutic effect is confined to the topical engagement of the mucosal epithelium. HPPCLS is distinct from standard sucralfate in its physical ability to maintain elevated surface concentrations of sucralfate. At 3 hours post-administration to rabbits with acetic acid-induced colitis (25), HPPCLS (polymerized sucralfate) maintains a surface concentration of sucralfate on normal mucosal lining that is 800% greater than that of non-polymerized standard sucralfate (of the same dose strength) and 2,400% greater on ulcerated or inflamed mucosal lining. It is assumed by the authors that reversal of mucosal immune activation associated with IBS (5-7) is likely involved in some manner (direct or indirectly) with the topical engagement of HPPCLS and the enteric mucosa.
The proposed model, therefore, bring to forefront a few basic givens. Firstly, mucosal immune activation is involved with the generation of all symptoms and signs of classic IBS. Immune activation occurs due to disturbance of the mucosal homeostasis. Thus, there is a genomic controlled primal drive to restore any perturbations of mucosal homeostasis and inflammation [which includes pro-inflammatory (PI) and anti-inflammatory (AI) agents] is the prime physiologic tool to restore homeostasis disrupted by an epithelial assault. Inflammation subsides only after a signal of “restored homeostasis” is received by the genome (26). Secondly, HPPCLS is topical and non-systemic agent. Thus, the HPPCLS mechanism of action infers the existence of an apical epithelial mediated process that is physically accessible to HPPCLS. Thirdly, given its topical mode of action, the chemical determinants of HPPCLS may point to epithelial-associated substances, ligands or receptors exposed to the luminal contents and accessible to HPPCLS. Fourthly, since there is complete cessation of the signs and symptoms of IBS in 2–3 days of using HPPCLS, then there must exist as well a network of submucosal feedback mechanisms satisfied by a particular epithelial event transpiring when HPPCLS adheres to the intestinal lining of the IBS patient. These four tenets form the basis of the model presented in this report.
Chemical determinants affecting HPPCLS adherence to the mucosa
Chemical determinants drive interactions between HPPCLS and the mucosal lining. Without these interactions there can be no clinical effects. Obviously the chemical determinants within HPPCLS define interplay between it and the mucosal lining (27). Chemical determinants in HPPCLS results from combining sucralfate and calcium chelated hydroxybutanedioate (CCHBD) (25). Basic science experiments involving flame photometry, measurements of turbidity and pH, and gravimetric analysis highlight key characteristics of HPPCLS. Low-solubility sucralfate is readily suspended by the addition of CCHBD, creating polymerization and sucralfate cross-linkage. This suspension of CCHBD-treated sucralfate is prolonged and self-sustained. The initial neutral pH of low-solubility sucralfate becomes acidic with the addition of CCHBD. This implies that the suspended material is negatively charged. Previously low-solubility sucralfate assumes a suspended amorphous appearance.
There are chemical determinants inherent to the mucosal lining itself. Certain mucosal characteristics are pertinent to understanding the HPPCLS-mucosal interaction. The adherence of HPPCLS, largely electronegative, is influenced by the preexisting electrostatic texture of the mucosal lining. Adherence of charged macromolecules to the mucosal lining was investigated by Jubeh et al. (27) using liposomes that were positively, negatively, and neutrally charged in colitis-induced rat intestine. Negatively charged liposomes adhered to positively charged areas of the mucosal lining. Liposomes that were positively charged adhered to negatively charged areas of the lining. Neutrally charged liposomes adhered evenly throughout the lining. Negatively charged areas were normal appearing non-inflamed mucosa. Positively charged areas were notably inflamed. This electrostatic texture mediates the adherence of the HPPCLS and the resulting clinically effects. Low-solubility sucralfate, changed by chelated calcium into an amorphous electronegative polymerized and cross-linked macromolecule, is known to hyper-accumulate on inflamed lining. The fact that it accumulates 7 times greater than expected on non-inflamed lining and 23 times greater on inflamed lining (25), implies that the cross-linked polymerization can persist intralumenally, possibly promoting pi-stacked accumulation of sucralfate on the mucosal lining.
Conceptual function-diagram of proposed model
The macro-elements of HPPCLS-mediated elimination of IBS can be conceptually depicted and diagrammed. The proposed model assumes that the existing mucosal immune activation associated with all forms of IBS (constipation and diarrhea dominant), as well as other FGIDs, indicate a breach of epithelial homeostasis and the ongoing attempts of the mucosa to restore itself under the continual presence of the offending intraluminal agent, whatever that agent may be. The model accommodates the possibility that there are no specific “offending agent(s)” but abnormal function of epithelial elements tasked with maintaining normal mucosal integrity. In other words, the proposed model accommodates the possibility that constituents of normal luminal contents can be misidentified as offensive by a defective epithelial surveillance, thus igniting immune activation commonly observed in the mucosa of IBS patients.
Having this, the macro-elements of HPPCLS-mediated elimination of IBS may have the following sequence of emergence: (I) a surface point of contact of “offending agent” or of misidentification of normal intraluminal agent as offensive; (II) triggered dispatch of transepithelial signal(s) to the nucleus to initiate inflammation and (III) multiple signal relays transmitted through the cytoplasm of the “offended” enterocyte to its nucleolus, relays that coordinate numerous messages received as result of the epithelial disturbance. To illustrate this theoretical engagement (Figure 1), the model depicts the disease activity as a hub of responses and actions. The model assumes that mucosal homeostasis is an evolutionary center maintained by a constitutively controlled balance of PI and AI agents of both the innate and adaptive immune system.
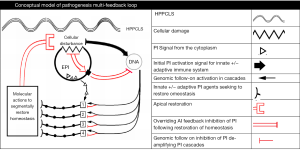
A signal (or signals) of epithelial injury or disturbance (whether apical, basal or cytoplasmal in location) from the injured (or disturbed) cells is relayed to its DNA which in turn incites a PI reaction complete with commensurate counterbalancing AI actions in an attempt to restore homeostasis. The ratio of PI to AI is determined by the extent of epithelial injury communicated from the epithelial cell to its genome.
The genome controls all cascades of PI/AI actions amplifying them to levels determined by the extent of injury (or perceived injury). If injury is minimal then a primary level of PI/AI response is initiated and controlled by the genome until it receives signal either verifying restored homeostasis or continued disturbance. If homeostasis is not achieved within a reasonable time or if continued injury is sustained, then the genome can amplify the PI/AI response, deepening the amplification as is required by the assault (or perceived assault). Once the epithelial breach is repaired, then a signal of “restored homeostasis” is relayed to the genome, which in turn de-amplifies its PI/AI actions. If the HPPCLS observations of rapid reversal of IBS are to be believed, then this de-amplification is segmental (occurring in the anatomical site of the gut), sequential and rapid.
Law of mucosal homeostatic equilibrium—proposed model by components
General principles governing the model
The conceptual model above is useful to outline the sequence of general events, but it omits cellular and humoral signaling molecules and the general principles governing their involvement. Inflammation is an act of preservation. If unsuccessful in restoring homeostasis, inflammation can become immunopathologic (26). It is a complex network of immunologic events under strict genomic control and modulation. During inflammation, cytokines, or signaling molecules, arise from cellular elements of the innate and adaptive immune system and provide exogenomic coordination. Based on the observed clinical effects of HPPCLS, it seems that the entire sequences of immune events are deactivated in an orderly fashion with the eventual outcome of restored baseline homeostasis of the epithelium.
It is the authors opinion that probiotics-associated substances, probably short chain fatty acids (SCFA) and other topically acting agents follow the same overall inhibitory AI trek but by way of differing mucosal pathways. The HPPCLS pathway is likely transforming growth factor/epithelial growth factor (TGF/EGF) or trefoil factor (TFF) dependent (28-34) while SCFA generated by probiotic microbes utilize TGF/EGF independent pathways (35-38). To identify relevant touch points for prospective analysis, the plausible compartments of tissue-level actions and counteractions should be assigned to known cellular, subcellular, autocrine and paracrine elements. Comparative analysis of the homeostatic concentrations with the concentration of these elements in patients with varied severity and types of IBS will permit elucidation of pathophysiology of IBS and FGIDs.
Regardless of the manner of mucosal-mediation used by either HPPCLS or probiotics, the cohorts of molecular and cellular mediators involved originate from both the innate and adaptive immune systems. Each anatomical layer of the GI lining (epithelial-applied mucus, the five cell-types populating the epithelium, the sub-epithelium and lamina propria) (39,40) play host to cellular and molecular mediators of both the innate and the adaptive immune systems—mediators that can be either PI and AI. In addition to this, the state of homeostasis is maintained by a genomic-controlled balance of PI and AI mediators with every genomic-initiated PI action accompanied by a commensurate counterbalancing AI reaction.
Interplay of specific components of the model
Armed with these general principles, a model of can be constructed comprising of known PI and AI mediators localized (I) to the lumen; (II) underneath the mucus blanket; (III) within and just beneath the epithelial lining and (IV) within the lamina propria. Figure 2 shows the dynamic of the proposed pathobiology of IBS and most functional bowel disorders.
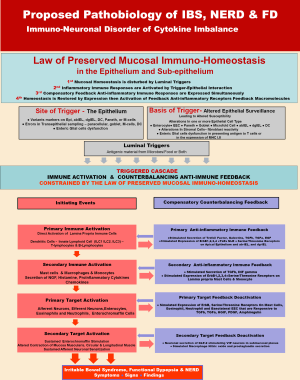
According to this model there are (I) luminal triggers; (II) activation of a PI immune response; (III) concomitantly expressed compensatory feed-back AI reaction; with (IV) homeostasis either immediately restored or delayed and finally, if homeostasis is delayed there is (V) a step-wise amplification of deepen PI immune response with its compensatory feed-back AI reaction. Prolonged delay of homeostasis gives rise to (VI) the signs, symptoms and findings associated with IBS and functional bowel disorders.
The model specifically identifies potential humoral and cellular elements possibly involved in successive amplification of the inflammatory immune response.
Pathobiology of IBS
The biology of the immune response comprises cellular and molecular elements that facilitate events required to mount an immune response. Alterations in any of these elements are pathologic, compromising the immune response, delaying restoration of homeostasis and thereby giving rise to the signs, symptoms and findings associated with IBS or other functional GI disorders.
Altered surveillance—site and basis of an immune trigger
Prior to any perturbation or disturbance of epithelial homeostasis and normal clinical function, there is an actively supported state of homeostatic surveillance (41,42). An equilibrium or neutral state of quiescence is maintained by cellular and molecular elements responsible for active and effectual surveillance. An intact immune response capable of restoring homeostasis once it is disturbed begins with effectual surveillance (43-46). A compromise in the cellular and molecular elements responsible for surveillance will play out as a compromised immune response, unable to maintain a stable homeostasis.
The trigger site for an immune response is the epithelium and the basis of an inept immune response can initiated from altered epithelial surveillance being either molecularly hyper-hypo-vigilant. Altered epithelial surveillance may spring from atypical alterations in the function of one or more epithelial cell types. Disordered variants in molecular markers on the alpha-beta or delta-gamma intra-epithelial lymphocytes (IELs) regionally distributed from the esophagus and the rectum (47-49) can compromise epithelial surveillance. Surveillance can be compromised by dysfunction or altered molecular markers on surfaces of epithelial enterocytes, enterochromaffin cells, Paneth or microfold cells, as each cell type contribute to homeostatic surveillance.
Errors in trans-epithelial sampling of luminal contents by paracellular, globlet, microfold and dendritic cells can compromise surveillance as well (40). Finally enteric glial cell dysfunction in presenting to T cells, luminal antigens previously processed by any of the afore-mentioned frontline epithelial cells can compromise surveillance. Compromised surveillance or breached epithelium will trigger primary immune activation.
Primary immune activation & compensatory AI immune reaction
Breached epithelium or compromised surveillance will trigger a primary immune activation within lamina propria immune cells, dendritic cells, or innate lymphocytes (T- and B-cells) secreting interleukins (ILC1, ILC2 and ILC3) (50,51). Compensatory AI immune reaction tasked with restoration of epithelial homeostasis involves secretion of TFFs, galectins, TGFβ, TGFα, and EGF. Primary immune activation also stimulates the apical expression on the epithelium of AI receptors (ErbB1, 2, 3, 4, TLR and NLR, serine/threonine receptors). Compensatory reactions also include reactionary activation of alpha-beta or delta-gamma IELs, chiefly responsible for epithelial surveillance.
Secondary immune activation and its compensatory AI immune reaction
As shown in Figure 2, should primary activation of the innate immune response with its compensatory AI reaction, fail to restore homeostasis, then secondary immune activation occurs, accompanied by its compensatory AI immune reaction. Distressed epithelial cells (such as upregulated enterocytes, enterochromaffins, Paneths, microfold cells, goblet or dendritic cells) activate submucosal mast cells, macrophages and monocytes to secrete nerve growth factor, histamine and PI cytokines (52-54). Compensatory reaction counterbalancing secondary immune activation involve stimulated secretion of TGFβ, interferon-γ, with membrane expression of AI receptors (ErbB1, 2, 3, 4, TLR and NLR, serine/threonine) on mast cells and monocytes in the lamina propria.
Primary target activation & compensatory primary target feedback deactivation
Primary target of secondary immune activation are afferent and efferent neurons (55,56), enterocytes, eosinophils, neutrophils and enterochromaffin cells (57-59).
Up-regulation of these targeted cells lead to membrane expression of AI receptors (ErbB1, 2, 3, 4, TLR and NLR, serine/threonine) on the effector cells and on recruited cells (mast cells, eosinophils, neutrophils) and on the basolateral aspect of enterochromaffin cells (6). These AI receptors are responsive to local concentrations of TGFβ, TGFα, HGIF, PDGF and amphiregulin (60-64).
Secondary target activation & compensatory secondary target feedback deactivation
Sustained, unabated stimulation of up-regulated afferent neurons, efferent neurons and enterochromaffin cells result in altered contraction of the mucosa muscularis, circular and longitudinal muscles as well as sustained afferent neuronal sensitization leading to pain and hyperalgesia (65,66). Neuro-cytokines and effector substances released by up-regulated neurons can (I) stimulate epithelial cells (esp. enterochromaffin cells) to secrete fluids (12,13,67) giving rise to diarrhea-dominant IBS; (II) stimulate sub-mucosal muscularis and the circular muscles of the gut to contract while simultaneously causing the longitudinal muscles to relax (68,69)—discordant contractions giving rise to constipation-dominant IBS, (such actions also result in intestinal cramping and bloating) and (III) stimulate capillary vessels to expand and increase their flow (12,13). Additionally, stimulated sub-mucosal sensory neurons release pain substances within the sub-mucosa and into the bloodstream; they also transmit up-regulating neuronal signals outside the GI tract into dorsal root ganglia of the spine to affect segments of the GI (12,13) that is proximal and distal to the area of immune activation. Activation of secondary targets lead to the clinical symptoms, signs and findings associated with IBS and other FGIDs. Counterbalancing these targeted activations are compensatory neuronal secretion of glucagon-like peptide-2 (GLP) which in turn stimulates VIP receptors in neurons within the submucosal plexus to modulate against the gut effects stemming from activation of secondary targets (70-73) In fact, GLP1 given to patients with IBS provides an effective, on demand, relief of acute pain attacks (74).
Summary
In IBS (as well as other FGIDs) it is likely that luminal triggers engage vulnerable elements of surveillance in the mucosal epithelium. Sites of vulnerability can be antigen-sampling systems involving paracellular, transepithelial cells (microfold cells and goblet cells), Paneth cells, dendritic cells or intraepithelial lymphocytes. This breach of epithelial homeostasis activates the primary (innate) and then secondary (cellular, adaptive) immune systems that in turn, up-regulate primary and secondary targets of PI activation. The inflammatory actions of up-regulated targets, though counterbalanced with compensatory AI feedback (in terms of membrane expression of AI markers or secretion of AI messengers), result in the symptoms, signs and findings of functional bowel syndrome. If no substantial defects exist in the mediating components of the primary and secondary immune systems, then topical actions that reverse the initial breach will provide a feedback inhibition signal (shown in red in Figure 1) sufficient to induce the genome to initiate successive deactivation of subservient immune cascades and to thereby reverse the signs and symptoms of the functional bowel syndrome.
Whether or not the clinical and experimental observations characteristic of IBS (altered tight junction, activated immune responses, etc.) are actually eliminated by HPPCLS remains unknown. It would be expected that molecular findings typical of IBS should be minimized, substantially reduced and or significantly subdued so as to offer negligible clinical consequences.
Conclusions
Deciphering the pathophysiology of any disorder is greatly benefited by the use of an intervention that dramatic ameliorates the signs and symptoms of disease. In the regulatory approval of interventions for IBS, the FDA requires a 12-week comparison with placebo with patient-reported (rather than clinician-reported) outcomes centered on (I) alleviation of pain/discomfort and (II) improved regulatory of bowel habits and consistency of stool. Instead the required 84 days of observation to verify a significant treatment effect, HPPCLS meet the patient-reported outcome-standard within 2–3 days. The resultant rate ratio is 14–56 times that expected with other interventions—lubiprostone, rifaximin, linaclotide, alosetron, tegaserod—and highlights HPPCLS’s mechanism of action as a possible window into the pathophysiology of functional bowel disorders. Being non-systemic and topically active, HPPCLS implicates mucosal determinants of IBS that are physically accessible. The model in concept (Figure 1) and in its specifics (Figure 2) provides several touch points of analysis to help pinpoint the true disturbance in IBS. If substantiated but others, HPPCLS-mediated reversal of IBS, implicate repairable breach or breaches in epithelial surveillance may give rise to the symptoms and signs of IBS and FGIDs.
Acknowledgements
None.
Footnote
Conflicts of Interest: R McCullough is an employee of Mueller Medical International owner of technology underlying high potency cross-linked sucralfate. J McCullough has no conflicts of interest to declare.
References
- Drossman DA, Hassler WL. Rome IV-Functional GI Disorders: disorders of Gut-Brain Interaction. Gastroenterology 2016;150:1257-61. [Crossref] [PubMed]
- Shih DQ, Kwan LY. All Roads Lead to Rome: Update on Rome III Criteria and New Treatment Options. Gastroenterol Rep 2007;1:56-65. [PubMed]
- Piche T. Tight junctions and IBS – the link between epithelial permeability, low grade inflammation, and symptom generation? Neurogastroenterol Motil 2014;26:296-302. [Crossref] [PubMed]
- Barbara G, Stanghellini V, de Giorgio R, et al. Activated mast cells in proximity to colonic nerves correlate with abdominal pain in irritable bowel syndrome. Gastroenterology 2004;126:693-702. [Crossref] [PubMed]
- Berin MC, Mckay DM, Perdue MH. Immune-epithelial interactions in host defense. Am J Trop Med Hyg 1999;60:16-25. [PubMed]
- Chadwick VS, Chen W, Shu D, et al. Activation of the mucosal immune system in irritable bowel syndrome. Gastroenterology 2002;122:1778-83. [Crossref] [PubMed]
- Walker MM, Talley NJ. Duodenal mastocytosis, eosinophilia and intraepithelial lymphocytosis as possible disease markers in the irritable bowel syndrome and functional dyspepsia. Aliment Pharmacol Ther 2009;29:765-73. [Crossref] [PubMed]
- Coates MD, Mahoney CR, Linden DR, et al. Molecular defects in mucosal serotonin content and decreased serotonin reuptake transporter in ulcerative colitis and irritable bowel syndrome. Gastroenterology 2004;126:1657-64. [Crossref] [PubMed]
- Mazzawi T, Gundersen D, Hausken T, et al. Increased chromogranin a cell density in the large intestine of patients with irritable bowel syndrome after receiving dietary guidance. Gastroenterol Res Pract 2015;2015:823897.
- Gershon MD, Tack J. The serotonin signaling system: from basic understanding to drug development for functional GI disorders. Gastroenterology 2007;132:397-414. [Crossref] [PubMed]
- Spiller RC. Effects of serotonin on intestinal secretion and motility. Curr Opin Gastroenterol 2001;17:99-103. [Crossref] [PubMed]
- Holzer P, Michil T, Danzer M. Surveillance of the gastrointestinal mucosa by sensory neurons. J Physiol Pharmacol 2001;52:505-21. [PubMed]
- Holzer P. Gastrointestinal pain in functional bowel disorders: sensory neurons as novel drug targets. Expert Opin Ther Targets 2004;8:107-23. [Crossref] [PubMed]
- Simrén M, Barbara G, Flint HJ, et al. Intestinal microbiota in functional bowel disorders: a Rome foundation report. Gut 2013;62:159-76. [Crossref] [PubMed]
- Weaver KR, Sherwin LB, Walitt B, et al. Neuroimaging the brain-gut axis in patients with irritable bowel syndrome. World J Gastrointest Pharmacol Ther 2016;7:320-33. [Crossref] [PubMed]
- Mayer EA, Naliboff BD, Craig AD. Neuroimaging of the brain-gut axis: from basic understanding to treatment of functional GI disorders. Gastroenterology 2006;131:1925-42. [Crossref] [PubMed]
- Glasziou P, Chalmers I, Rawlins M, et al. When are randomized trials unnecessary? Picking signal from noise. BMJ 2007;334:349-51. [Crossref] [PubMed]
- Howick J, Friedmann C, Tsakok M, et al. Are treatments more effective than placebos? A systematic review and meta-analysis. PLoS One 2013;8:e62599. [Crossref] [PubMed]
- McCullough RW. Rapid (48–96 Hour) Symptom-Sign Relief of ROME III Irritable Bowel Syndrome (IBS), Functional Heartburn (FH) & Postprandial Distress Syndrome (PDS) Using Cross-Linked Polyanionic Saccharides: An Implication for Pathophysiology. Gastroenterology 2013;144:S-932-S933. [Crossref]
- McCullough RW. Mucosa-Centric Clinical Effects of High Potency Sucralfate: 28 Day 83% Resolution of Undifferentiated Dyspepsia, 28 Day 83% Reversal of Sign & Symptoms of Co-Morbid IBS and 1 Week 80% Healing of GERD Erosions -Results of Two Randomized Clinical Trials. Gastroenterology 2014;146:S-263. [Crossref]
- Chang L, Heitkemper MM, Wiley JW, et al. 2015 James W. Freston Single Topic Conference: A Renaissance in the Understanding and Management of Irritable Bowel Syndrome. Clin Gastroenterol Hepatol 2016;14:e77-86. [Crossref] [PubMed]
- Tarnawski A, Hollander D, Krause WJ, et al. Does sucralfate affect the normal gastric mucosa? Histologic, ultrastructural, and functional assessment in the rat. Gastroenterology 1986;90:893-905. [Crossref] [PubMed]
- Tarnawski A, Hollander D, Stachura J, et al. Effect of sucralfate on the normal human gastric mucosa. Endoscopic, histologic, and ultrastructural assessment. Scand J Gastroenterol Suppl 1987;127:111-23. [Crossref] [PubMed]
- Hollander D, Tarnawski A, Krause WJ, et al. Protective effect of sucralfate against alcohol-induced gastric mucosal injury in the rat. Macroscopic, histologic, ultrastructural, and functional time sequence analysis. Gastroenterology 1985;88:366-74. [Crossref] [PubMed]
- Kashimura K, Ozawa K. Sucralfate Preparations. US Patent 5,968,906. Oct19, 1999.
- Ashley NT, Weil ZM, Nelson RJ. Inflammation: mechanisms, costs and natural variation. Annu Rev Ecol Evol Syst 2012;43:385-406. [Crossref]
- Jubeh TT, Barenholz Y, Rubinstein A. Differential adhesions of normal and inflamed rat colonic mucosa by charged liposomes. Pharmaceutical Research 2004;21:447-53. [Crossref] [PubMed]
- McCullough RW. Saccharide compositions and method of use. US Patent 7,795,239. September 14, 2010.
- Konturek SJ, Konturek JW, Brzozowski T, et al. Chapter 17. Effects of sucralfate on growth factor availability. In: Hollander D, GNJ Tygat GN. editors. Sucralfate: From basic science to the bedside. New York: Plenum Press, 1995:175-89.
- Werner S, Grose R. Regulation of wound healing by growth factors and cytokines. Physiol Rev 2003;83:835-70. [PubMed]
- Sturm A, Dignass AU. Epithelial restitution and wound healing in inflammatory bowel disease. World J Gastroenterol 2008;14:348-53. [Crossref] [PubMed]
- Harris RC, Chung E, Coffey RJ. EGF receptor ligands. Exp Cell Res 2003;284:2-13. [Crossref] [PubMed]
- Andresen JL, Ehlers N. Chemotaxis of human keratocytes is increased by platelet-derived growth factor-BB, epidermal growth factor, transforming growth factor-alpha, acidic fibroblast growth factor, insulin-like growth factor-I, and transforming growth factor-beta. Curr Eye Res 1998;17:79-87. [Crossref] [PubMed]
- Dignass A, Lynch-Devaney K, Kindon H, et al. Trefoil peptides promote epithelial migration through a transforming growth factor beta-independent pathway. J Clin Investig 1994;94:376-83. [Crossref] [PubMed]
- Taupin DR, Kinoshita K, Podolsky DK. Intestinal trefoil factor confers colonic epithelial resistance to apoptosis. Proc Natl Acad Sci U S A 2000;97:799-804. [Crossref] [PubMed]
- Asarat M, Apostolopoulos V, Vasiljevic T, et al. Short-chain fatty acids regulate cytokines andTh17/Treg cells in human peripheral blood mononuclear cells in vitro. Immunol Invest 2016;45:205-222. [Crossref] [PubMed]
- Rolandelli RH, Koruda MJ, Settle RG, et al. Effects of intraluminal infusion of short-chain fatty acides on the healing of colonic anastomosis in rat. Surgery 1986;100:198-204. [PubMed]
- Scheppach W. Effects of short chain fatty acids on gut morphology and function. Gut 1994;35:S35-S38. [Crossref] [PubMed]
- Wang J, Friedman EA. Short chain fatty acids induce cell cycle inhibitors in colonocytes. Gastroenterology 1998;114:940-6. [Crossref] [PubMed]
- Shaykhiev R, Bals R. Interactions between epithelial cells and leukocytes in immunity and tissue homeostasis. J Leukoc Biol 2007;82:1-15. [Crossref] [PubMed]
- Öhman L, Tornblom H, Simren M. Crosstalk at the mucosal border: importance of the gut microenvironment in IBS. Nat Rev Gastroenterol Hepatol 2015;12:36-49. [Crossref] [PubMed]
- Mowat AM, Agace WW. Regional specialization within theintestinal immune system. Nat Rev Immunol 2014;14:667-85. [Crossref] [PubMed]
- Serhan CN, Savill J. Resolution of inflammation: the beginning programs the end. Nat. Immunol 2005;6:1191-7. [Crossref] [PubMed]
- Sears BF, Rohr JR, Allen JE, et al. The economy of inflammation: when is less more? Trends Parasitol 2011;27:382-7. [Crossref] [PubMed]
- Singh V, Agrewala JN. Regulatory of pro-Th 1 and pro-Th2 cytokines in modulating the activitiy ofTh1 andTh2 cells when B cell and macrophages are used as antigen presenting cells. BMC Immunolog 2006;7:7-17. [Crossref]
- Sheridan BS, Lefrancois L. Intraepithelial lymphocytes: to serve and protect. Curr Gastroenterol Rep 2010;12:513-21. [Crossref] [PubMed]
- Stadnyk AW. Intestinal epithelial cells as a source of inflammatory cytokines and chemokines. Can J Gastroenterol 2002;16:241-6. [Crossref] [PubMed]
- Regnault A, Kourilsky P, Cumano A. The TCR-beta chain repertoire of gut-derived T lymphocytes. Semin Immunol 1995;7:307-19. [Crossref] [PubMed]
- Camerini V, Panwala C, Kronenberg M. Regional specialization of the mucosal immune system. Intraepithelial lymphocytes of the large intestine have a different phenotype and function than those of the small intestine. J Immunol 1993;151:1765-6. [PubMed]
- Lundqvist C, Baranov V, Hammarstrom S, et al. Intra-epithelial lymphocytes. Evidence for regional specialization and extrathymic T cell maturation in the human gut epithelium. Int Immunol 1995;7:1473-87. [Crossref] [PubMed]
- Lundqvist C, Baranov V, Sodersterom K, et al. Phenotype and cytokine profile of intraepithelial lymphocyte. Ann N Y Acad Sci 1995;756:395-9. [Crossref] [PubMed]
- Land WG. The role of damage-associated molecular patters in human disease. Part 1 – promoting inflammation and immunity. Sultan Qaboos Univ Med J 2015;15:e9-e21. [PubMed]
- Land WG. The role of damage-associated molecular patters in human disease. Part 2 – DAMPs as diagnostics, prognostics and therapeutics in clinical medicine. Sultan Qaboos Univ Med J 2015;15:e157-70. [PubMed]
- Mohamadzadeh M, Luftig R. Dendritic cells: in the forefront of immunopathogenesis and vaccine development – A review. J Immune Based Ther Vaccines 2004;2:1. [Crossref] [PubMed]
- McKay DM, Perdue MH. Intestinal epithelial function: the case for immuno-physiological regulation. Implications for disease (Part 1). Dig Dis Sci 1993;38:1377-87. [Crossref] [PubMed]
- McKay DM, Perdue MH. Intestinal epithelial function: the case for immuno-physiological regulation. Implications for disease (Part 2). Dig Dis Sci 1993;38:1735-45. [Crossref] [PubMed]
- Opal SM, DePalo VA. Anti-inflammatory cytokines. Chest 2000;117:1162-72. [Crossref] [PubMed]
- Leon A, Buriani A, Dal-Toso R, et al. Mast cells synthesize, store, and release nerve growth factor. Proc Natl Acad Sci USA 1994;91:3739-43. [Crossref] [PubMed]
- Bauer O, Razin E. Mast cell-nerve interactions. News Physiol Sci 2000;15:213-8. [PubMed]
- Spiller RC. Inflammation as a basis for functional GI disorders. Best Pract Res Clin Gastroenterol 2004;18:641-61. [Crossref] [PubMed]
- Barbara G, Degiorgio R, Stanghellini V, et al. A role for inflammation in irritable bowel syndrome? Gut 2002;51:i41-i44. [Crossref] [PubMed]
- Blikslager AT, Moeser AJ, Gookin JL, et al. Restoration of barrier function in injured intestinal mucosa. Physiol Rev 2007;87:545-64. [Crossref] [PubMed]
- Janeway CA Jr, Travers P, Walport M, et al. Immunobiology: The Immune System in Health and Disease. 5th Edition. New York: Garland Science, 2001.
- Kaplan HJ, Niederkorn JY. Regional immunity and immune privilege. Chem. Immunol. Allergy 2007;92:11-26. [Crossref] [PubMed]
- Mosmann TR, Coffman RL. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol 1989;7:145-73. [Crossref] [PubMed]
- Nathan C. Points of control in inflammation. Nature 2002;420:846-52. [Crossref] [PubMed]
- Tørring N, Jørgensen PE, Sørensen BS, et al. Increased expression of heparin binding EGF (HB-EGF), amphiregulin, TGF alpha and epiregulin in androgen-independent prostate cancer cell lines. Anticancer Res 2000;20:91-5. [PubMed]
- Mayer EA, Gebhart GF. Basic and clinical aspects of visceral hyperalgesia. Gastroenterology 1994;107:271-93. [Crossref] [PubMed]
- Gebhart GF. Visceral pain- peripheral sensitization. Gut 2000;47 Suppl 4:iv54-iv55. [Crossref] [PubMed]
- Gershon MD. Nerves, reflexes, and the enteric nervous system: pathogenesis of the irritable bowel syndrome. J Clin Gastroenterol 2005;39 Suppl 3:S184-93. [Crossref] [PubMed]
- Simrén M, Stotzer PO, Sjövall H, et al. Abnormal levels or neuropeptide Y and peptide YY in colon in irritable bowel syndrome. Eur J Gastroenterol Hepatol 2003;15:55-62. [Crossref] [PubMed]
- Fox-Threlkeld JA, Daniel EE, Christinck F, et al. Peptide YY stimulates circular muscle contractions of the isolated perfused canine ileum by inhibiting nitric oxide release and enhancing acetylcholine release. Peptides 1993;14:1171-8. [Crossref] [PubMed]
- Zhao P, Han Y. Low incidence of positive smooth muscle antibody and high incidence of isolated IgM elevation in Chinese patients with autoimmune hepatitis and primary biliary cirrhosis overlap syndrome: a retrospective study. BMC Gastroenterol 2012;12:1. [Crossref] [PubMed]
- Hellström PM, Hein J, Björnsson E, et al. Clinical trial: the glucagon-like peptide-1 analogue ROSE-010 for management of acute pain in patients with irritable bowel syndrome: a randomized, placebo-controlled, double-blind study. Aliment Pharmacol Ther 2009;29:198-206. [Crossref] [PubMed]
Cite this article as: McCullough R, McCullough J. Deciphering the pathophysiology of irritable bowel syndrome and functional gastrointestinal disorders—an alternative model for pathogenesis: cytokine controlled transepithelial multi-feedback loop. Transl Gastroenterol Hepatol 2017;2:18.


