Lymphadenectomy: state of the art
Introduction
The lymphatic route is the main way of spread of gastric cancer (GC), and lymph node involvement is the main prognostic factor after potentially curative (R0) resection (1-3). The systematic removal of regional lymph nodes (lymphadenectomy) has always been considered a crucial step in GC surgery, and lymph node dissection as a direct measure of the quality of surgery. However, the benefit of extended (D2) lymphadenectomy remained a matter of debate for long time, above all in Western countries, in light of the results of the Dutch and British randomized studies, which showed no evidence of overall survival benefit after D2 dissection compared with the D1, at the expenses of much higher postoperative mortality rates (4,5). These results were much discordant as compared with observational data from specialized Western centers, which reported low complications rates and postoperative mortality risk, with high long-term survival rates even in advanced nodal stages (6-9). Some clarifications were provided by the re-evaluation of long-term results of the Dutch trial, which showed a reduction of locoregional recurrence and GC-related deaths, above all in the long-term, in patients treated by D2 lymphadenectomy (10,11). Nowadays, most recent treatment guidelines from East and West advice D2 dissection as the standard treatment of resectable GC in non-early stage, above all in patients deemed as medically fit, and treated in specialized centers with ad adequate volume of GC surgery and appropriate surgical expertise and postoperative care (12-17).
Material and methods
A literature review was performed using Medline/PubMed and Cochrane Library with the following key words: GC, stomach neoplasm, lymphadenectomy, node dissection. The language used for the research was English.
Rationale of extended lymphadenectomy
The rationale of the potential clinical benefit of lymphadenectomy in GC is based upon four main cornerstones: (I) increase in the number of removed nodes and adequate disease staging; (II) removal of potentially metastatic nodes and increase in surgical radicality; (III) reduction of GC recurrence, above all locoregional; (IV) potential improvement in long-term survival.
Increase in the number of removed nodes and adequate disease staging
It is well known that extended lymphadenectomy is associated with an increase in the number of removed lymph node, compared with mode limited lymph node dissections. This is an important point for disease staging, as UICC/AJCC TNM classification advice a minimum of 16 lymph nodes to be analysed for a correct nodal staging (pN). Some specific studies demonstrated statistically the potential improvement of extended lymphadenectomy in this aspect. The analysis of data from specialized centers belonging to the Italian Research Group for Gastric Cancer (GIRCG) showed that D1 dissection is associated with a rate of inadequate staging (less than 16 removed nodes) in 54.4% of patients. On the contrary, these rates decreased to 6.2% and 1.4%, respectively, in patients submitted to D2 or D3 lymphadenectomy, which are then associated with an adequate disease staging in the majority of cases (18).
Removal of potentially metastatic nodes and increase in surgical radicality
It is intuitive, but also clearly demonstrated in all studies dealing with this issue, that the more lymph node are removed, the more positive nodes are found (19,20). This implies that the extended lymph node dissection is associated with an increase in surgical radicality, even if this is not directly measurable case by case. Several studies calculated the incidence of lymph node metastases in second level nodes, removed by D2 dissection. The rates of nodal metastasis in second-level nodes (8a, 10, 11p/d, 12a) range according to depth of invasion, histotype, tumor location and other pathological factors (9,20-23). It is evident that removing these metastatic nodes could favour an increase in surgical radicality. Furthermore, as a survival benefit of extended lymphadenectomy has been supposed even in node-negative patients, removal of potential micrometastasis in these nodes could also improve oncological outcome (24,25).
Reduction of GC recurrence
The increase in surgical radicality obtained with extended lymphadenectomy could be associated with a reduction in tumor recurrence, with special reference to the locoregional failure. The re-evaluation of the Dutch trial demonstrated a significant decrease of GC-related deaths in patients submitted to D2 vs. D1 (37% vs. 48%, respectively), whereas death due to other diseases was similar in both groups. Local recurrences were 22% in the D1 group vs. 12% in D2, and regional recurrences were 19% in D1 vs. 13% in D2 (11). If we look at survival curves of this study, it is evident that after 2–3 years from operation the cumulative risk of death due to GC is increased in the D1 group, and several long-term tumor-related deaths even after 5 years of follow-up are observed. In the GIRCG experience, a reduction of locoregional recurrence has been observed with time, with a parallel increase in the quality of lymphadenectomy and the number of removed lymph nodes (26).
Potential improvement in long-term survival
No long-term survival benefit was observed, in Dutch and British randomized trials, in patients treated by D2 dissection. However, this may be due, at least in the Dutch trial, to the initial gap of higher postoperative mortality in the D2 group. Both trials revealed much higher mortality and morbidity after D2 lymphadenectomy when compared with the D1 arm. High complication rates could be explained by both the high number of participating centers, not all of which specialized in this type of surgery, and by the inappropriate design of the trials, which included routine distal pancreatectomy and splenectomy in the D2 procedure. The 15-year follow-up evaluation of the Dutch trial showed a trend to overall survival improvement in D2 group, although not statistically significant; if we could virtually exclude this initial gap, long-term survival difference may be much higher, probably statistically significant (11). In the Italian trial by Degiuli et al., better long-term outcome was observed in patients with N2 tumors treated by D2 dissection compared with D1; in such trial, postoperative mortality was overlapping in the D1 and D2 groups (27). Then, it is probable that D2 dissection, when performed in specialized, high-volume centers, could offer a long-term survival benefit if postoperative complications and mortality are not increased. This is confirmed by the results of the Taiwan randomized trial comparing limited and extended lymph node dissection carried out at a single specialized institution in Taiwan (28). This trial demonstrated a survival benefit in overall and disease free survival rates after extended lymph node dissection, with limited postoperative complications.
D3 (D2 plus) lymphadenectomy
Lymph node dissection in “posterior” (8p, 12b/p, 13) and para-aortic stations (16 a2/b1) is currently not included in the “standard” D2 lymphadenectomy, according to the guidelines of the Japanese Gastric Cancer Association (JGCA). This because the final long-term results of the JCOG 9501 randomized trial comparing D2 with D2 plus para-aortic lymphadenectomy failed to show an overall survival benefit for the super-extended group (29). However, we should underline that: (I) in such trial patients with clinical metastases to para-aortic nodes were not eligible for randomization; as such, the survival benefit of D2 plus was denied when performed with a “prophylactic” intent, but a potential benefit in patients with distant node metastases could not be excluded; (II) several observational and phase II studies reported long-term survivors in patients with metastases to para-aortic nodes, when treated by D2 plus lymphadenectomy; these rates were particularly high when surgery is preceded by neoadjuvant chemotherapy (30-33); (III) the incidence of distant lymph node metastases in Western patients has been estimated to be higher than Asian series, because it is related, besides T stage, to proximal tumor location and diffuse Lauren histotype (34); (IV) the JCOG 9501 compared patients treated by D2 vs. D2 plus the dissection, but the surgical difference between the two groups was the removal of para-aortic nodes, whereas “posterior” stations (8p, 12b/p, 13) were similarly dissected in the two groups under study; as such, in our opinion the conclusions of the study do not justify the exclusion of “posterior” lymphadenectomy from surgical guidelines. For all these reasons, the GIRCG guidelines advice D2 plus lymphadenectomy in patients at risk of lymph node metastases to “posterior” and para-aortic nodes (17). These can be identified in advanced forms located in proximal third, and advanced diffuse histotype in the distal third for para-aortic lymphadenectomy; the results of an observational GIRCG study for the identification of groups at risk of metastases to “posterior” stations are going to be published. However, it is advisable that these procedures are performed in centers specialized with the D2 dissection, where more extended dissection could be performed safely, or in the setting of clinical studies.
Lymphadenectomy for early forms
Although endoscopic approach to early forms is increasing in specialized centers in the West, it is still far to become a clinical standard. Early forms not treatable by endoscopic resection should be submitted to surgical resection with lymphadenectomy. According to the JGCA treatment guidelines, D1 lymphadenectomy may be adequate in early forms with clinically negative lymph nodes (16). However, we should underline that a proportion of early forms in the West are of diffuse histotype, which is associated with a higher risk of lymph node metastases and greater lymph node spread, above all when submucosa is involved. Furthermore, in the West endoscopic resections, which can be considered as treatment but also staging procedures, are performed much less frequently than in East Asia, and the clinical diagnosis of lymph node metastasis by imaging procedures has still a low accuracy. As such, the GIRCG guidelines advise a standard D2 lymphadenectomy in early forms of GC (17). Only in selected cases (high-risk patients, early forms with favourable pathological characteristics, not treatable by endoscopic resections) more limited procedures should be considered (D1 plus). This includes the removal of perigastric lymph nodes (stations from 1 to 6), left gastric artery nodes (stations 7), celiac axis nodes (station 9), and hepatic artery (8a), according to the JGCA guidelines. Dissection along splenic artery (11p/d) depends upon the extent of gastrectomy (subtotal/total) for its inclusion in the D1 plus or D2 lymphadenectomy. Molecular characteristics of GC could indicate in the future new possibilities to select the extent of lymphadenectomy in high-risk patients, with special reference to microsatellite instability cases, which demonstrated a lower propensity to lymphnodal spread, particularly in the aged (35,36).
Early forms of GC could also be treated by minimally-invasive (laparoscopic or robotic) approach, which demonstrated non-inferior oncological results than open surgery in recent studies (37,38). However, it should be emphasized that oncological criteria regarding resection margin and lymph node dissection need to be carefully followed in minimally-invasive procedures.
Lymphadenectomy for advanced forms
The extent of lymphadenectomy is crucial in surgical treatment of advanced forms of GC. An essential condition is that good early postoperative results in terms of morbidity and mortality should be ensured. This is consistent with the reports of observational non-randomized studies from specialized centers (6-9,18,26-28).
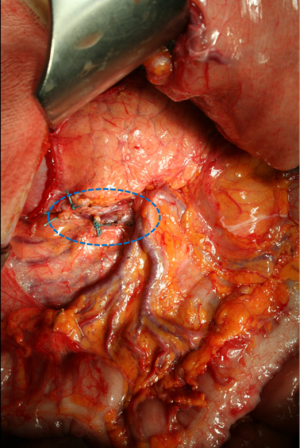
The correct procedure of lymphadenectomy involves the removal of nodal stations from 1 to 12, with some variations depending upon the extent of gastric resection. Special attention should be paid upon to the complete removal of infra-pyloric nodes (station 6), right paracardial nodes (station 1), left gastric artery nodes (station 7), celiac axis (station 9), hepatic artery (station 8a), splenic artery (station 11), and hepatoduodenal ligament nodes (station 12a). We emphasize the notable importance of infra-pyloric lymphadenectomy, because station 6 is frequently involved even in early forms of GC located in the distal third, and this station is located in the first level; as such, an inadequate clearance at this level involves an incomplete D1 (D0) lymphadenectomy. The removal of station 14v (mesenteric vein) is advisable when macroscopic involvement of station 6 is present (Figure 1). Posterior bursectomy, with the removal of the anterior sheet of transverse mesocolon and pancreatic capsule, is advisable in cT3/T4 tumors, above all when located in the posterior wall of the stomach.
As for indications to splenectomy, final survival analysis of a randomized controlled trial (JCOG0110), designed to evaluate the role of splenectomy in total gastrectomy for proximal GC which does not invade the greater curvature, demonstrated significant non-inferiority of spleen preservation (39). Total gastrectomy with splenectomy should be recommended for tumors that are located along the greater curvature or when a macroscopic involvement of stations 4sa or 10 is present.
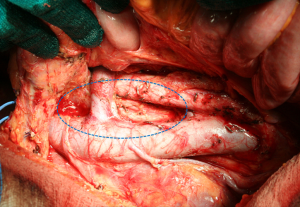
As previously stated, more extended lymphadenectomies (D2 plus) could be performed in selected cases at risk of metastasis to posterior (8p, 12p, 12b, 13), or para-aortic (16a2, b1) lymph nodes, in specialized centers and in the setting of clinical studies. Para-aortic lymphadenectomy should be limited to 16 a2/b1 groups (Figure 2). Proximal tumors or diffuse type tumors are particularly prone to metastasize to distant nodes, and in our opinion they may benefit from a super-extended lymphadenectomy (30,33,34,40). A recent GIRCG study reported a decrease of locoregional recurrence in diffuse type tumors treated by D2 plus dissection, as compared with the standard D2 (41).
Early results
The correct procedure of extended lymphadenectomy requires specialized experience in gastro esophageal surgery with an appropriate learning curve; indeed, the complication and mortality rates reported in the specialized Western centers are limited, generally overlapping or slightly higher than those reported by the Japanese authors (6-9,18,26-28,42,43). Furthermore, it is particularly important to limit the use of splenopancreatic resection to only selected cases. Splenopancreatic resection, besides not providing a proven benefit in terms of survival, considerably increases postoperative complications, as demonstrated in the Dutch and British randomized trials (4,5). Indeed, in the Italian trial by Degiuli et al., D2 dissection did not increase postoperative complications and mortality when spleen and pancreatic tail are preserved (43). It is also worthy of note that in the British trial resection of spleen and pancreas were found to have a significant negative influence on overall survival (5). In the GIRCG experience, data collected from a prospective database of 2,822 patients, most of which submitted to extended lymphadenectomy (>15 removed nodes in 78% of cases, >25 nodes in 53%) postoperative mortality was 3.5%, even when including aged patients, advanced stages and so on (26). These data indicate that extended lymphadenectomy can be performed safely in Western patients when adopting correct criteria and with an adequate surgical volume. Anyway, some risk factors for morbidity, linked to patient characteristics, have been identified in different studies: advanced age (particularly after 75 years), presence of important co-morbidities (mainly cirrhosis and renal failure), high co-morbidity index, hypoalbuminemia and poor nutritional status are those more frequently associated with advanced grade of postoperative complications after gastrectomy with lymphadenectomy (42,44-47). A characteristic of specialized centers is the ability to prompt identification and management of severe complications, which is associated with a significant decrease of their lethality rate.
Long-term results
Long-term survival rates after extended lymphadenectomy change notably in literature according to different series. Interpretation of the results from randomized trials indicate: (I) good long-term results after both D1 and D2 dissection, which were higher than expected; this was probably due to the selection of cases for the trial, but also in part to the standardization of the technique, as well as to frequent cases of contamination, which resulted in the removal of second-level stations in the D1 arm (43,48). Indeed, a recent evaluation performed on the Dutch trial revealed a significant survival benefit of the D2 over D1 when excluding cases with non-compliance or contamination (49); (II) the survival benefit after extended lymphadenectomy becomes evident after 2–3 years from operation, as observed in the Dutch and the Taiwan trial (11,28). The subset of patients with N2 disease in the Italian trial show similar clinical behaviour (43). This may be due to the potential reduction of locoregional failures, which are associated with longer timing of recurrence.
Generally, good long-term survival rates are reported in observational studies after extended lymphadenectomy in specialized centers from both Eastern and Westerns countries (6-9,19,21,33,41,43). In particular, some studies reported that with the implementation of lymphadenectomy procedures with time, a progressive reduction of locoregional recurrences has been observed (26). However, it should be emphasized that when comparing long-term results from Eastern and Western series, a significant better outcome at the same stage is observed in the former, suggesting potential biological differences related to the tumor, to the ethnicity or to the immunological system (50-54). When analysing long-term results in Western large series, it is evident that survival rates at advanced pT (serosal involvement) and pN stages (N3a and N3b) are unsatisfactory, even when submitting patients to extended lymphadenectomy (55). As such, in these stages a multidisciplinary approach is advisable, with possible integrated approach including neoadjuvant treatments or intraperitoneal chemotherapy (56). Recent studies reported very good long-term survival rates in patients with extensive or bulky lymph node metastases submitted to neoadjuvant chemotherapy and D2 plus lymphadenectomy (31,57). This is particularly true in Western series, where the rate of diffuse type tumors, associated with a high propensity to spread to regional and distant nodes, is increasing with time (26,56).
Conclusions
After years of extensive clinical and scientific research, hundreds of studies, clinical trials, meta-analysis and review papers, in many cases with contrasting and contradictory results, some fixed points seem to be reached about lymphadenectomy for GC. D2 dissection is now recognized in most guidelines all over the world as the standard treatment for resectable GC. More limited dissections could be applied for selected cases (early forms not treatable by endoscopic resections, high-risk patients), whereas more extended lymphadenectomy (D2 plus) could be indicated in advanced forms with high risk of metastases to distant nodes, but in specialized centers or in the setting of clinical studies. The integration with neoadjuvant therapies and multimodality treatments could offer a chance of cure in groups of patients with still poor results when approached with standard treatment.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Marrelli D, Morgagni P, de Manzoni G, et al. External Validation of a Score Predictive of Recurrence after Radical Surgery for Non-Cardia Gastric Cancer: Results of a Follow-Up Study. J Am Coll Surg 2015;221:280-90. [Crossref] [PubMed]
- Han DS, Suh YS, Kong SH, et al. Nomogram predicting long-term survival after d2 gastrectomy for gastric cancer. J Clin Oncol 2012;30:3834-40. [Crossref] [PubMed]
- Kattan MW, Karpeh MS, Mazumdar M, et al. Postoperative nomogram for disease-specific survival after an R0 resection for gastric carcinoma. J Clin Oncol 2003;21:3647-50. [Crossref] [PubMed]
- Bonenkamp JJ, Hermans J, Sasako M, et al. Extended lymph-node dissection for gastric cancer. N Engl J Med 1999;340:908-14. [Crossref] [PubMed]
- Cuschieri A, Weeden S, Fielding J, et al. Patient survival after D1 and D2 resections for gastric cancer: long-term results of the MRC randomized surgical trial. Surgical Co-operative Group. Br J Cancer 1999;79:1522-30. [Crossref] [PubMed]
- de Manzoni G, Verlato G, Guglielmi A, et al. Prognostic significance of lymph node dissection in gastric cancer. Br J Surg 1996;83:1604-7. [Crossref] [PubMed]
- Siewert JR, Böttcher K, Stein HJ, et al. Relevant prognostic factors in gastric cancer: ten-year results of the German Gastric Cancer Study. Ann Surg 1998;228:449-61. [Crossref] [PubMed]
- Roukos DH, Lorenz M, Encke A. Evidence of survival benefit of extended (D2) lymphadenectomy in western patients with gastric cancer based on a new concept: a prospective long-term follow-up study. Surgery 1998;123:573-8. [Crossref] [PubMed]
- Roviello F, Marrelli D, Morgagni P, et al. Survival benefit of extended D2 lymphadenectomy in gastric cancer with involvement of second level lymph nodes: a longitudinal multicenter study. Ann Surg Oncol 2002;9:894-900. [Crossref] [PubMed]
- Hartgrink HH, van de Velde CJ, Putter H, et al. Extended lymph node dissection for gastric cancer: who may benefit? Final results of the randomized Dutch gastric cancer group trial. J Clin Oncol 2004;22:2069-77. [Crossref] [PubMed]
- Songun I, Putter H, Kranenbarg EM, et al. Surgical treatment of gastric cancer: 15-year follow-up results of the randomised nationwide Dutch D1D2 trial. Lancet Oncol 2010;11:439-49. [Crossref] [PubMed]
- Lee JH, Kim JG, Jung HK, et al. Clinical practice guidelines for gastric cancer in Korea: an evidence-based approach. J Gastric Cancer 2014;14:87-104. [Crossref] [PubMed]
- Martin-Richard M, Custodio A, García-Girón C, et al. Seom guidelines for the treatment of gastric cancer 2015. Clin Transl Oncol 2015;17:996-1004. [Crossref] [PubMed]
- Moehler M, Baltin CT, Ebert M, et al. International comparison of the German evidence-based S3-guidelines on the diagnosis and multimodal treatment of early and locally advanced gastric cancer, including adenocarcinoma of the lower esophagus. Gastric Cancer 2015;18:550-63. [Crossref] [PubMed]
- Smyth EC, Verheij M, Allum W, et al. Gastric cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2016;27:v38-v49. [PubMed]
- Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer 2016. [Epub ahead of print].
- De Manzoni G, Marrelli D, Baiocchi GL, et al. The Italian Research Group for Gastric Cancer (GIRCG) guidelines for gastric cancer staging and treatment: 2015. Gastric Cancer 2017;20:20-30. [PubMed]
- Verlato G, Roviello F, Marchet A, et al. Indexes of surgical quality in gastric cancer surgery: experience of an Italian network. Ann Surg Oncol 2009;16:594-602. [Crossref] [PubMed]
- Kong SH, Lee HJ, Ahn HS, et al. Stage migration effect on survival in gastric cancer surgery with extended lymphadenectomy: the reappraisal of positive lymph node ratio as a proper N-staging. Ann Surg 2012;255:50-8. [Crossref] [PubMed]
- Smith DD, Nelson RA, Schwarz RE. A comparison of five competing lymph node staging schemes in a cohort of resectable gastric cancer patients. Ann Surg Oncol 2014;21:875-82. [Crossref] [PubMed]
- Sasako M, McCulloch P, Kinoshita T, et al. New method to evaluate the therapeutic value of lymph node dissection for gastric cancer. Br J Surg 1995;82:346-51. [Crossref] [PubMed]
- Di Leo A, Marrelli D, Roviello F, et al. Lymph node involvement in gastric cancer for different tumor sites and T stage: Italian Research Group for Gastric Cancer (IRGGC) experience. J Gastrointest Surg 2007;11:1146-53. [Crossref] [PubMed]
- Lee SL, Lee HH, Ko YH, et al. Relevance of hepatoduodenal ligament lymph nodes in resectional surgery for gastric cancer. Br J Surg 2014;101:518-22. [Crossref] [PubMed]
- Siewert JR, Kestlmeier R, Busch R, et al. Benefits of D2 lymph node dissection for patients with gastric cancer and pN0 and pN1 lymph node metastases. Br J Surg 1996;83:1144-7. [Crossref] [PubMed]
- Baiocchi GL, Tiberio GA, Minicozzi AM, et al. A multicentric Western analysis of prognostic factors in advanced, node-negative gastric cancer patients. Ann Surg 2010;252:70-3. [Crossref] [PubMed]
- Marrelli D, Pedrazzani C, Morgagni P, et al. Changing clinical and pathological features of gastric cancer over time. Br J Surg 2011;98:1273-83. [Crossref] [PubMed]
- Degiuli M, Sasako M, Ponti A, et al. Randomized clinical trial comparing survival after D1 or D2 gastrectomy for gastric cancer. Br J Surg 2014;101:23-31. [Crossref] [PubMed]
- Wu CW, Hsiung CA, Lo SS, et al. Nodal dissection for patients with gastric cancer: a randomised controlled trial. Lancet Oncol 2006;7:309-15. [Crossref] [PubMed]
- Sasako M, Sano T, Yamamoto S, et al. D2 lymphadenectomy alone or with para-aortic nodal dissection for gastric cancer. N Engl J Med 2008;359:453-62. [Crossref] [PubMed]
- Hasegawa S, Yoshikawa T, Rino Y, et al. Priority of lymph node dissection for Siewert type II/III adenocarcinoma of the esophagogastric junction. Ann Surg Oncol 2013;20:4252-9. [Crossref] [PubMed]
- Tsuburaya A, Mizusawa J, Tanaka Y, et al. Neoadjuvant chemotherapy with S-1 and cisplatin followed by D2 gastrectomy with para-aortic lymph node dissection for gastric cancer with extensive lymph node metastasis. Br J Surg 2014;101:653-60. [Crossref] [PubMed]
- Tokunaga M, Ohyama S, Hiki N, et al. Can superextended lymph node dissection be justified for gastric cancer with pathologically positive para-aortic lymph nodes? Ann Surg Oncol 2010;17:2031-6. [Crossref] [PubMed]
- Roviello F, Pedrazzani C, Marrelli D, et al. Super-extended (D3) lymphadenectomy in advanced gastric cancer. Eur J Surg Oncol 2010;36:439-46. [Crossref] [PubMed]
- de Manzoni G, Di Leo A, Roviello F, et al. Tumor site and perigastric nodal status are the most important predictors of para-aortic nodal involvement in advanced gastric cancer. Ann Surg Oncol 2011;18:2273-80. [Crossref] [PubMed]
- Marrelli D, Polom K, Pascale V, et al. Strong Prognostic Value of Microsatellite Instability in Intestinal Type Non-cardia Gastric Cancer. Ann Surg Oncol 2016;23:943-50. [Crossref] [PubMed]
- Polom K, Marrelli D, Roviello G, et al. Molecular key to understand the gastric cancer biology in elderly patients-The role of microsatellite instability. J Surg Oncol 2016. [Epub ahead of print]. [Crossref] [PubMed]
- Hyun MH, Lee CH, Kim HJ, et al. Systematic review and meta-analysis of robotic surgery compared with conventional laparoscopic and open resections for gastric carcinoma. Br J Surg 2013;100:1566-78. [Crossref] [PubMed]
- El-Sedfy A, Brar SS, Coburn NG. Current role of minimally invasive approaches in the treatment of early gastric cancer. World J Gastroenterol 2014;20:3880-8. [Crossref] [PubMed]
- Sano T, Sasako M, Mizusawa J, et al. Randomized controlled trial to evaluate splenectomy in total gastrectomy for proximal gastric carcinoma (JCOG0110): Final survival analysis. J Clin Oncol 2015;33 suppl 3;abstr 103.
- Kim KT, Jeong O, Jung MR, et al. Outcomes of Abdominal Total Gastrectomy for Type II and III Gastroesophageal Junction Tumors: Single Center's Experience in Korea. J Gastric Cancer 2012;12:36-42. [Crossref] [PubMed]
- de Manzoni G, Verlato G, Bencivenga M, et al. Impact of super-extended lymphadenectomy on relapse in advanced gastric cancer. Eur J Surg Oncol 2015;41:534-40. [Crossref] [PubMed]
- Marrelli D, Pedrazzani C, Neri A, et al. Complications after extended (D2) and superextended (D3) lymphadenectomy for gastric cancer: analysis of potential risk factors. Ann Surg Oncol 2007;14:25-33. [Crossref] [PubMed]
- Degiuli M, De Manzoni G, Di Leo A, et al. Gastric cancer: Current status of lymph node dissection. World J Gastroenterol 2016;22:2875-93. [Crossref] [PubMed]
- Marrelli D, Roviello F, De Stefano A, et al. Surgical treatment of gastrointestinal carcinomas in octogenarians: risk factors for complications and long-term outcome. Eur J Surg Oncol 2000;26:371-6. [Crossref] [PubMed]
- Jeong SH, Ahn HS, Yoo MW, et al. Increased morbidity rates in patients with heart disease or chronic liver disease following radical gastric surgery. J Surg Oncol 2010;101:200-4. [PubMed]
- Yang JY, Lee HJ, Kim TH, et al. Short- and Long-Term Outcomes After Gastrectomy in Elderly Gastric Cancer Patients. Ann Surg Oncol 2017;24:469-477. [PubMed]
- Rausei S, Ruspi L, Rosa F, et al. Extended lymphadenectomy in elderly and/or highly co-morbid gastric cancer patients: A retrospective multicenter study. Eur J Surg Oncol 2016;42:1881-1889. [Crossref] [PubMed]
- Bonenkamp JJ, Hermans J, Sasako M, et al. Quality control of lymph node dissection in the Dutch randomized trial of D1 and D2 lymph node dissection for gastric cancer. Gastric Cancer 1998;1:152-159. [Crossref] [PubMed]
- de Steur WO, Hartgrink HH, Dikken JL, et al. Quality control of lymph node dissection in the Dutch Gastric Cancer Trial. Br J Surg 2015;102:1388-93. [Crossref] [PubMed]
- Strong VE, Song KY, Park CH, et al. Comparison of gastric cancer survival following R0 resection in the United States and Korea using an internationally validated nomogram. Ann Surg 2010;251:640-6. [Crossref] [PubMed]
- Strong VE, Wu AW, Selby LV, et al. Differences in gastric cancer survival between the U.S. and China. J Surg Oncol 2015;112:31-7. [Crossref] [PubMed]
- Marrelli D, Roviello F. Prognostic difference between Eastern and Western patients with gastric cancer: quality of care, ethnicity, or biology? Ann Surg 2014;259:e78-9. [Crossref] [PubMed]
- Lin SJ, Gagnon-Bartsch JA, Tan IB, et al. Signatures of tumour immunity distinguish Asian and non-Asian gastric adenocarcinomas. Gut 2015;64:1721-31. [Crossref] [PubMed]
- Marrelli D, Polom K, Roviello F. Ethnicity-related differences in tumor immunity: a new possible explanation for gastric cancer prognostic variability? Transl Gastroenterol Hepatol 2016;1:11. [Crossref]
- Marrelli D, Morgagni P, de Manzoni G, et al. Prognostic value of the 7th AJCC/UICC TNM classification of noncardia gastric cancer: analysis of a large series from specialized Western centers. Ann Surg 2012;255:486-91. [Crossref] [PubMed]
- Marrelli D, Polom K, de Manzoni G, et al. Multimodal treatment of gastric cancer in the west: Where are we going? World J Gastroenterol 2015;21:7954-69. [PubMed]
- Ito S, Sano T, Mizusawa J, et al. A phase II study of preoperative chemotherapy with docetaxel, cisplatin, and S-1 followed by gastrectomy with D2 plus para-aortic lymph node dissection for gastric cancer with extensive lymph node metastasis: JCOG1002. Gastric Cancer 2016. [Epub ahead of print]. [Crossref] [PubMed]
Cite this article as: Marrelli D, De Franco L, Iudici L, Polom K, Roviello F. Lymphadenectomy: state of the art. Transl Gastroenterol Hepatol 2017;2:3.