Report of one case: surgical treatment for neuroendocrine tumors
Disease history
A 68-year-old female patient was admitted due to “decreased appetite and upper abdominal pain for 2 months”. In February 2014, the patient experienced decreased appetite and upper abdominal pain, without any apparent cause. In April 2014, she visited our hospital, during which the abdominal CT displayed a space-occupying lesion in spleen. The patient had no condition/symptom such as fever, jaundice, diarrhea, constipation, skin flushing, or skin ecchymosis in recent years. She had disease histories of hypertension and diabetes. Her father died of esophageal cancer. Physical examination did not reveal any positive sign.
Preoperative auxiliary examinations
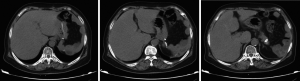
Abdominal CT plain scans (on April 8, 2014, in our hospital)
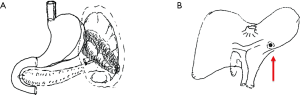
The spleen tumor involved the greater curvature of stomach, with blurred border with the tail of pancreas (Figure 1). The largest cross section sized 10.7 cm × 7.4 cm. A malignant change was considered. Among the peritoneal nodules, some were in tortuous vessels and some might involve the lymph nodes.
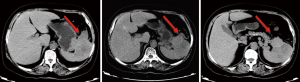
Abdominal MRI plain scans (on April 29, 2014, in our hospital)
The mass was located in the anterior part of the spleen, with irregular morphology and blurred margin (Figure 2). Part of the mass invaded the greater curvature of stomach and part of it had blurred margin with the tail of pancreas. The largest cross section sized 9.4 cm × 6.9 cm. It showed intermediate and high signals on T2WI/FS, which corresponded to restricted diffusion on DWI sequences.
Tumor marker detection (on April 11, 2014, in our hospital)
The detection showed that the CA19-9, CA242, and CEA levels were normal.
Preoperative diagnosis
- A splenic space-occupying mass, with a high possibility of being malignant;
- Hypertension;
- Type 2 diabetes mellitus.
Diagnosis and treatment
Based on the disease histories, symptoms, and auxiliary examination findings, a malignant tumor was suspected, which was indicated for a surgery. Under general anesthesia, exploratory laparotomy + resection of the body and tail of pancreas + resection of part of stomach wall + removal of nodules on liver surface were performed on May 5, 2014.
Intraoperative exploration
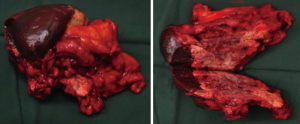
No effusion or implanted/metastatic nodule was seen in abdominal and pelvic cavities. The tumor was located inside the spleen, sized about 12 cm × 10 cm. The tumor protruded the spleen at the lower splenic pole and splenic hilum and invaded the splenic portion of the mesentery of the colon, the stomach wall at the gastric greater curvature (sized about 6.0 cm × 5.0 cm), and the tail of pancreas. In addition, a small nodule was found beneath the liver capsule at the left side of the round ligament of liver (Figure 3). The nodule had a yellow surface and sized about 0.6 cm × 0.5 cm. After it was resected, rapid intraoperative pathology revealed that it was a tumor; however, whether it was a primary tumor or a metastatic tumor (or tumor embolus) required further differentiation.
Surgical procedures
The gastrocolic ligament and splencolic ligament were separated to thoroughly expose the pancreas and spleen. Areas near the splenic hilum and the tail of pancreas were carefully dissected to separate and ligate the splenic artery and vein and several short gastric vessels. The splenic artery and vein, tissues in the tail of pancreas, the invaded gastric wall tissue, and short gastric vessels at the upper splenic pole were divided one by one using an endoscopic linear cutter/stapler (Echelon), so as to completely remove the spleen (and the tumor), part of stomach wall, and tissue in the tail of pancreas (Figure 4). The operation was smooth. The abdominal cavity was flushed after surgery. The amounts of equipment were correct. After complete hemostasis, the abdominal drainage tube was indwelled; then, the incision was closed layer-by-layer.
Postoperative recovery
Due to the wide and severe surgical trauma, the patient was monitored and cared in ICU after the surgery. The muscle strength of right limbs decreased on the first postoperative day. Ischemic cerebral vascular disease was considered after neurological consultations. The condition was improved after symptomatic treatment including anticoagulant therapy, antihypertensive therapy, and intravenous infusion of dextran. The patient was returned to the normal ward on day 4 and discharged on day 10.
Postoperative pathology
- (Pancreatic body and tail + fundus of stomach + spleen): neuroendocrine tumors, with mitotic figures <1/10 HPF and Ki-67 <1% (+). The tumor was mainly located in spleen, involving pancreatic gland, gastric muscular wall, adipose tissue in splenic hilum, and adipose tissue around the pancreas (colonic mesentery). The resection margin was negative;
- In the left liver, focal infiltration of hepatic tumor tissue was seen in liver tissue, which met the diagnostic criteria of a metastatic lesion;
- In our current case, the tumor morphology and immunohistochemical results were consistent with those of a low grade (G1) tumor. However, the tumor was large in size, with wide involvement, suggesting poor biological behavior;
- Immunohistochemical findings: AE1/AE3(3+), CK18(3+), ChrA(1+), Syn(2+), CD56(3+), S100(2+), and Ki-67<1%(+).
Postoperative follow-up
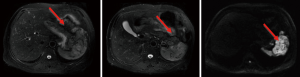
No obvious relapse had been found till April 2015 (Figure 5).
Discussion
Sites of gastroenteropancreatic neuroendocrine neoplasms (GEP-NENs)
GEP-NENs can occur in any part of the digestive system. In 2012, Guo et al. reviewed all the relevant Chinese literature published between 1954 and 2011 and identified a total of 11,671 GEP-NEN cases, among which pNENs (n=5,807, 49.8%) were most common (1). The majority of pNENs was functional pNENs (n=5,205, 89.6%), among which insulinoma predominated (n=4,962, 85.4%). Meanwhile, the misdiagnosis rate of GEP-NENs was as high as 55.1%. Another article retrospectively analysed the 178 GEP-NENs cases treated in the First Affiliated Hospital of Sun Yet-sun University and also found that pNENs were most common (n=62, 34.8%), followed by rectal NENs (n=36, 20.2%) (2). In addition, NENs have also been reported in liver, kidney, bladder, mesentery, bladder, and appendix (3). In our current case, while the primary lesion was located inside the spleen, it also invaded the stomach wall and the tail of pancreas, with hepatic metastasis. Thus, it was an NEN that had occurred in a rare site. Therefore, the sites of NENs can be diverse; in addition to the common primary tumors in pancreas and gastrointestinal tract, the possibility of misdiagnosis should be avoided. Different tumors have different prognoses, and a misdiagnosis can prevent the patients from benefiting from specific treatment.
Surgical treatment of liver metastasis
Similar to other gastrointestinal malignancies, liver is also the most common site of pNEN metastasis. If surgery can remove 90% of the lesion, concurrent or staged resection of the primary and metastatic lesions can be considered (4). Achieving R0/R1 resection requires the following conditions: (I) well-differentiated G1/G2 tumors; (II) without extra-abdominal metastasis or diffuse peritoneal metastasis; (III) without right cardiac dysfunction; and (III) for patients with positive octreotide scan results (i.e., with somatostatin receptor), peptide receptor targeted radiotherapy (PRRT) may be applied following a cytoreductive surgery. The 5-year survival rate was 47–76% after surgical treatment, which is higher than that (30–40%) in non-surgical patients; however, the recurrence rate can be as high as 76%, mainly localized to the liver (5,6). Similar to pNENs, liver is also the most common site for GI-NENs metastases. For GI-NENs patients with liver metastasis, resection of liver metastasis may be considered. Resection (R0/R1) of liver metastasis from NENs of midgut and hindgut origin may achieve encouraging long-term survival. Up to now study has investigated NENs in other rare sites.
Prognosis
In 2011, Wang et al. (7) found that the 1-, 3-, and 5-year survival rates of 52 patients were 76.9%, 54.2%, and 41.7%, respectively after medium- and long-term follow-up. During the follow-up, 13 patients (25.0%) died, among whom 2 died due to multiple complications and nosocomial infections after surgery and 11 died of postoperative tumor relapse/metastasis. Patients with pathologically confirmed NET had better prognosis than those with neuroendocrine carcinoma and mixed adenoneuroendocrine carcinoma. The median survivals of patients with pathologically confirmed G1, G2, and G3 NENs were 8.7, 2.0, and 3.0 years, showing significant difference. The median survivals of patients with or without distant metastasis were 2.1 and 8.3 years, respectively, also showing significant difference. Compared with other malignancies, GEP-NENs typically progress slowly and have relatively good prognosis: the 5-year survival rate was 50.4%; even when metastasis occurs, the 5-year survival rate still reaches 21.8%. Therefore, the pathological type, grade, and distant metastasis of GEP-NENs are correlated with survival.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Guo L, Tang C. Current Status of Clinical Research on Gastroenteropancreatic Neuroendocrine Tumors in China. Chinese Journal of Gastroenterology 2012;17:276-8.
- CSCO Neuroendocrine Neoplasms Expert Committee. China gastrointestinal pancreatic neuroendocrine tumor expert consensus 2013. Chinese Clinical Oncology 2013;18:815-32.
- Zhang J, Wang X, Hu C, et al. The Imaging Features of Neuroendocrine Tumors in the Rare Region. Journal of Clinical Radiology 2011;30:989-93.
- Guettier JM, Kam A, Chang R, et al. Localization of insulinomas to regions of the pancreas by intraarterial calcium stimulation: the NIH experience. J Clin Endocrinol Metab 2009;94:1074-80. [Crossref] [PubMed]
- Touzios JG, Kiely JM, Pitt SC, et al. Neuroendocrine hepatic metastases: does aggressive management improve survival? Ann Surg 2005;241:776-83; discussion 783-5. [Crossref] [PubMed]
- House MG, Cameron JL, Lillemoe KD, et al. Differences in survival for patients with resectable versus unresectable metastases from pancreatic islet cell cancer. J Gastrointest Surg 2006;10:138-45. [Crossref] [PubMed]
- Wang Y. Comparision of Clinical and Pathological Features Between SPT and PET and the Significance of Secretagogin in Differential Diagnosis. Available online: http://cnki.sgst.cn/KCMS/detail/detail.aspx?filename=1011057976.nh&dbcode=CMFD&dbname=CMFD2011
Cite this article as: Hu H, Zhao H. Report of one case: surgical treatment for neuroendocrine tumors. Transl Gastroenterol Hepatol 2016;1:83.