Interventional therapy for rectal neuroendocrine tumor with liver metastases: report of one case
Case presentation
Brief history
A 42-year-old male patient was admitted to the Department of Interventional Therapy of our center on November 23, 2013 due to “altered bowel habit for 1 month and rectal neuroendocrine tumor with liver metastases found during health check-up 1 week ago”. On October 27, 2013, multiple space-occupying lesions were found by ultrasound during health check-up. Then the patient received abdominal magnetic resonance imaging (MRI) in Changhai Hospital, which revealed the presence of intrahepatic malignancies, which were considered to be metastatic lesions. “Thin” stools with reduced defecation frequency have been observed in the past one month. His body weight has decreased by 10 kg. There were no other special complaints. He had no previous history of relevant conditions.
Physical examination
A soft mass sized 2–3 cm with relatively clear border was palpable 5 cm above the anal verge.
Auxiliary examinations
On November 14, 2013, colonoscopy in our center revealed a 2-cm mass 5 cm above the anal verge. With a sunken center and erosive and necrotic surface, the mass was soft in texture and easy to bleed. The mass could be touched during the digital rectal examination. The large intestine showed no other abnormality.
On November 18, 2013, colonoscopic biopsy identified six gray-white corn-like tissues. A diagnosis of neuroendocrine tumor (5 cm away from the anal verge) was made. Although mitotic figure was difficult to find, the currently available biopsy tissues supported the diagnosis of a G1 tumor. Immunohistochemical findings included: SYN (+), CHG (partially +), CD56 (+), CKs (+), CK20 (−), CEA (−), CDX2 (−), Ki-67 (2% +), CD34 (−), CD117 (−), DOG-1 (−), HMB45 (−), S100 (−), A103 (−), CD68 (tissue cells +), and DES (−).
On November 23, 2013, further testing showed: NSE, 18.6 (normal range, <15.2 ng/mL); CA19-9, 11.3 U/mL (normal range, <37 U/mL); and chromogranin A (CgA), 29.18 ng/mL (normal range, <94 ng/mL). Other tumor markers showed no abnormality.
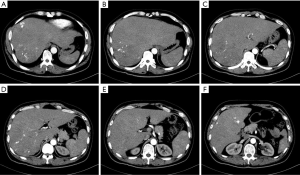
On November 18, 2013, CT (plain + contrast-enhanced) revealed: multiple hypodense lesions were seen inside the liver; contrast-enhanced CT showed that the lesions were remarkably enhanced during the arterial phase and the density was decreased during the portal phase; the maximum diameter was about 5.7 cm. No swollen lymph node was seen in the peritoneum. Metastatic malignancies in the liver were considered (Figure 1).
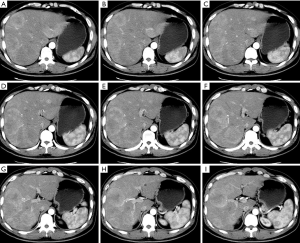
In this mid-aged male patient, rectal and hepatic space-occupying lesions were found during health check-up; rectal biopsy revealed neuroendocrine tumor (NET) (G1), along with multiple liver metastases with rich blood supply. The tumor burden exceeded 70%. As indicated by multidisciplinary treatment (MDT) consultations, the patient had lost the chance for radical surgery. Interventional therapy for liver metastasis and primary tumor of the rectum was then planned (Figures 2,3), during which liver biopsy would be performed to optimize the pathology for intrahepatic metastatic lesions.
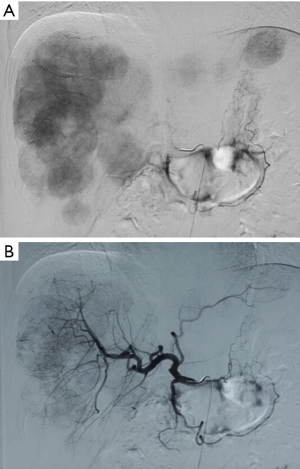

On November 25, 2013, liver biopsy + percutaneous transcatheter arterial chemoembolization (TACE) + transcatheter arterial infusion (TAI) chemotherapy were performed. Biopsy before the interventional therapy showed the presence of epithelial cell tumors in right anterior lobe, whereas mitotic figures were difficult to be seen. In combination with enzyme-labeling results [Ki-67 (5–10% +)], the findings met the diagnostic criteria of NET (G2). A rectal NET with multiple liver metastases was considered. Immunohistochemical findings included: CHG (+), SYN (+), Ki-67 (5–10% +), VILLIN (+), CDX-2 (−), SR2 (+), SR5 (+), ISL-1 (+), CD117 (−), EPCAM (+), and S100P (−).
The interventional therapy scheme and timing are shown in Table 1. The protocol included oxaliplatin, irinotecan, epirubicin and Lipiodol® Ultra Fluid. A total of 9 times of interventional therapy was performed from November 23, 2013 to August 1, 2015. After each intervention, tumor marker measurements and imaging were routinely performed to assess the effectiveness of interventional therapy (Figure 4).
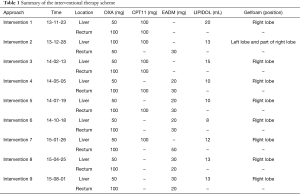
Full table
After the first intervention, a long-acting somatostatin analogue (octreotide acetate; 30 mg, im, qm) was added on December 1, 2013 and lasted for 8 months.
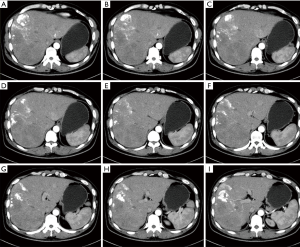
After the 9th intervention, CT performed on December 31, 2015 revealed that the multiple intrahepatic metastases remarkably shrank (partial response) and tumor biomarker level decreased than before (Figures 5,6), and the rectal lesion was still stable. Currently the patient was still under close follow-up.
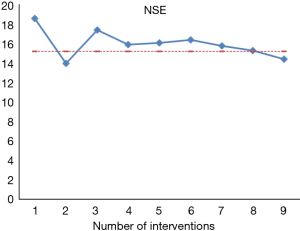
Thus, interventional therapy targeting liver metastases and primary lesion in patients with advanced rectal NET could control tumor progression and prolong the survival.
Discussion
Interventional therapy for rectal neuroendocrine tumor with liver metastases
Interventional therapy including radiofrequency ablation, arterial chemoembolization, and selective internal radiation therapy can be used as palliative treatment to control liver metastasis, reduce tumor burden, decrease hormone secretion, and thus improve the patients’ quality of life. While no large-scale prospective clinical tries have evaluated the specific efficacies of these local therapies on advanced rectal NETs with liver metastasis, these local therapies have been used in combination with systemic therapies (e.g., somatostatin analogues and mTOR inhibitors) in clinical settings. More data are needed for long-term outcome of interventional therapy for advanced rectal NETs. In addition, quality-of-life metrics should be incorporated in future comparative clinical studies and trials. We conclude that transarterial embolisation or transarterial chemoembolisation should be used as a treatment panel in predominant hepatic burden for indolent metastatic rectal NETs that have a long survival.
Mounting molecular analyses elucidate gene expression differences between primary tumor and metastases. Ki-67 expression heterogeneity is of relevance in tumors that are ultimately regarded of higher grade and malignant behavior. Hence, a recommendation should be made against multiple biopsies for Ki-67 assessment providing positive and comprehensive information on tumor disease.
Acknowledgements
Funding: The study was funded by National Natural Science Foundation of China (Nos. 81401923, 81572294); CSCO-Novartis neuroendocrine tumor development fund (2013).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Cite this article as: Liu L, Han X, Lou W. Interventional therapy for rectal neuroendocrine tumor with liver metastases: report of one case. Transl Gastroenterol Hepatol 2016;1:74.


