Glucagonoma syndrome: report of one case
Case presentation
Disease history
A 60-year-old male patient was admitted due to “recurrent systemic pruritus and erythema for 3 years, which worsened in the past 3 months, along with weight loss and diarrhoea”. Skin rashes in lower legs started 3 years ago and gradually involved the thighs, abdomen, lower back, head and face, and hands and feet. The patient had localized itchy skin first, followed by erythema, blisters, and ruptured scabs. The redness around these lesions further expanded and finally the scabs fell off. A diagnosis of “dermatitis” was made in a local hospital; however, the symptom was not alleviated after symptomatic treatment. High blood glucose was detected during the treatment and thus a diagnosis of “diabetes” was made. After oral administration with antidiabetic drugs, the fasting blood glucose levels fluctuated between 6 and 9 mmol/L. In the past 3 months, his symptoms worsened, along with anorexia and marked weight loss. His body weight had decreased by about 20 kg since disease onset. Other histories showed no special record.
Physical examination
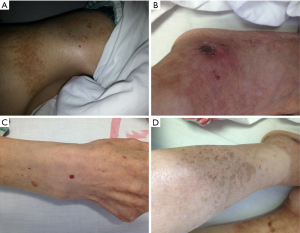
The patient had the appearance characteristic of anemia. His skin had a number of patchy erythema, along with the existence of scabs, scaling, pigmentation, and other morphologies (Figure 1), especially in hands/feet and around mouth/lips. A mass sized about 8.0 cm × 6.0 cm was palpable in the mid-left abdomen. The mass has clear margin and moderate texture. It was fixed in location and there was no tenderness.
Auxiliary examination
Auxiliary examination at admission showed that the chromogranin A (CgA) level was 492.0 ng/mL (the upper normal threshold: 94 ng/mL).
Also, measurements at admission showed the serum glucagon level was 648 ng/L and the neuron-specific enolase (NSE) level was 20.45 ng/mL (the upper normal threshold: 15.2 ng/mL).
Ultrasound at admission revealed a space-occupying lesion (about 5 cm in diameter) in the pancreas.
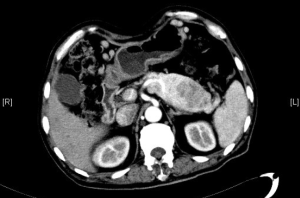
Contrast-enhanced abdominal CT at admission: The pancreatic head was found to have shrunk remarkably, and the body and tail of the pancreas enlarged, in which a slightly hypodense mass sized 5.3 cm × 8.5 cm was found. Contrast-enhanced CT showed that the lesion was heterogeneously enhanced; in the later stages, the internal localized enhancement decreased, whereas the central necrotic area showed no enhancement at all. The mass had blurred margin. Part of the lesion compressed the splenic artery and vein, with blurred local margin. It had the closest relationship with lateral joints outside the left suprarenal gland. There was a hypodense filling defect in the inferior vena cava, along with twisted blood vessels seen in the abdominal cavity (Figure 2).
Routine blood tests at admission: red blood cell (RBC) counts, 2.86×1012/L; hemoglobin, 84 g/L; protein, 22 g/L; and fasting glucose, 8.4 mmol/L. The carbohydrate antigen 19-9 (CA-199) and carcinoembryonic antigen (CEA) were normal.
Based on the above disease histories, symptoms/signs, and laboratory findings, the following clinical diagnoses were made: (I) a neuroendocrine tumor in the body and tail of the pancreas; (II) possible glucagonoma; and (III) necrolytic migratory dermatitis. Preoperative evaluation revealed that the tumor was resectable. Preoperative preparations included parenteral nutrition support, somatostatin analog treatment and inferior vena cava filter placement after admission. Then, resection of the body and tail of the pancreas combined with splenectomy was performed under general anesthesia, during which a tumor sized about 6.0 cm × 6.0 cm broke through the pancreatic capsule. The operation was smooth, and the intraoperative blood loss was about 100 mL.
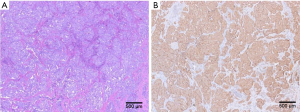
Postoperative pathology confirmed that the diagnosis was a neuroendocrine tumor involving the body and tail of pancreas and the spleen. The mitotic rate was about 1/10 HPF and the Ki-67 labeling index was 10%, which met the diagnostic criteria of a G2 lesion. Eight splenic hilar lymph nodes were detected, among which 3 were involved by tumor. Immunohistochemical findings: CK-Canton (++), CD56 (+), SYN (+++), CHG (+), KI67 (10% +), CD10 (−), CK7 (−), PR (90% +++), SSR2 (+++), SSR5 (+), C-met (90%+), and B-cat (membrane +) (Figure 3). Skin symptoms were remarkably resolved after surgery, along with good blood glucose control. Venography and filter removal were performed two weeks after filter placement. The whole courses passed evenly without severe complications and patient recovered well.
Discussion
Typical clinical manifestations of glucagonoma
- Necrolytic migratory erythema (NME): NME is the most characteristic clinical manifestation of glucagonoma and also the core diagnostic criteria in most cases. Typically the skin lesion lasts 7–14 days. Initially the lesion is characterized by erythema of varying shapes, along with a pale bulge at the center of erythema, showing herpes-like changes. After the lesion becomes ruptured, moist wound forms, around which exfoliation of epithelium can be observed. Sometimes it is covered with a layer of psoriasis-like skin scabs. While its center can be healed, its outer areas continue to expand to form margins with clear borders. After its healing, the skin still has brown pigment. The skin rashes are widely distributed throughout the body, occurring one after another, especially at perineum and limb extremities. During the disease onset, the histological changes are as follows: sudden necrolysis of one third of the epidermis, manifested as the thickening of spinous layer cells; and normal appearance of two thirds of the epidermis, shown as the clearly-defined border between the necrotic epidermis and the normal epidermis. Its pathogenetic mechanism remains unclear. It has been widely believed that the lesion might be caused by skin dystrophy due to low blood amino acid and zinc deficiency following the enhanced catabolism and gluconeogenesis due to increased serum glucagon. However, it has also been argued that the elevated pancreatic glucagon can induce the epidermis to produce a large number of arachidonic acid and its metabolites, thus causing skin inflammatory lesions;
- Diabetes mellitus: diabetes is seen in two thirds of glucagonoma patients. Generally, there is only mild diabetes or abnormal glucose tolerance. Non-insulin-dependent diabetes mellitus is more common, without obvious complication or ketoacidosis. It may be explained that the excessive quantities of glucagon may lead to glucose metabolism disorder; however, the serum glucagon level does not necessarily parallel with the diabetic level. Usually dietary adjustment or oral administration of hypoglycemic agents can control symptoms;
- Anemia: it is manifested as normochromic normocytic anemia, in which the bone marrow is normal or occasionally accompanied with poor red cell proliferation; the serum iron and folic acid levels can be normal. Thus, oral iron supplementation cannot improve anemia in such patients. The anemia may be associated with the wasting effect of the disease and/or result from the inhibited RBC prodcution due to the excessive quantities of glucagon;
- Marasmus: the body weight often remarkably decreases, which may be caused by high catabolism due to the glucagon, wasting effect of the tumor, and chronic diarrhea;
- Thrombopoiesis: about 25% of patients can also have deep vein thrombosis. All these five typical manifestations were observed in our current case. Other common symptoms include glossitis, cheilitis, diarrhea, neuropsychiatric symptoms, and accompanying endocrine disorders.
Perioperative management of glucagonoma
Preoperative testing of serum CgA and NSE is recommended. Change in serum CgA level can reflect the possible metastasis and recurrence of the tumor and also has an important prognostic value. For functioning glucagonoma, the corresponding hormone levels should also be tested before surgery. Symptoms caused by excessive hormone secretion should be controlled with drugs, if possible, before surgery. For glucagonoma, short-acting somatostatin receptor antagonists can be used to control hormone syndrome and correct fluid-electrolyte imbalances, which is particularly important to ensure the surgical safety. During the postoperative adjuvant therapy of glucagonoma, the long-term use of long-acting somatostatin analogue octreotide may be considered.
Lymph node dissection for glucagonoma
We believe lymph node involvement is a poor prognostic factor for the neuroendocrine tumors of the pancreas. Routine pancreatic resection combined with lymph node dissection is recommended for these tumors (except for insulinoma). Our current case underwent lymph node dissection, during which eight splenic hilar lymph nodes were detected and three were found to be with tumor metastasis.
Acknowledgements
Funding: National Natural Science Foundation of China (Nos. 81401923, 81572294); CSCO-Novartis neuroendocrine tumor development fund (2013).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Cite this article as: Han X, Wang D, Kuang T, Rong Y, Lou W. Glucagonoma syndrome: report of one case. Transl Gastroenterol Hepatol 2016;1:70.


