Gastric neuroendocrine neoplasm with liver metastases: a case report
Case presentation
History
A 39-year-old female was admitted due to “gastric neuroendocrine neoplasm (gNEN) with multiple liver metastases identified 9 months ago during the health check-up”. In September 2014, abdominal CT performed during health check-up in a local hospital showed the presence of a gastric mass and multiple liver space-occupying lesions; the patient had no symptom such as abdominal distension, abdominal pain, nausea, vomiting, or hematemesis. In October 2014, the patient visited our hospital, during which gastroscopy revealed an irregular ridge-like lesion at the lesser curvature of the stomach body (about 40 cm away from the incisor tooth). Pathological and immunohistochemical findings supported the diagnosis of a well-differentiated neuroendocrine tumor (G1). Abdominal MRI in our hospital suggested the possibility of multiple liver metastases and multiple perigastric lymph node metastases.
Personal and family medical history had no special record.
Examinations and diagnosis at admission
Physical examination
Physical examinations showed stable vital signs, no superficial lymph node was palpable throughout the body, no significant abnormalities by cardiopulmonary auscultation, soft abdomen without tenderness, no palpable liver or spleen below ribs, normal bowel sounds, and negative digital rectal examination (DRE) findings.
Auxiliary examination
- Laboratory tests: routine blood test, blood biochemistry, coagulation functions, viral markers, and tumor markers showed no obvious abnormality;
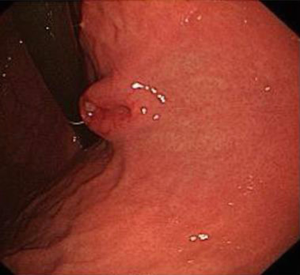
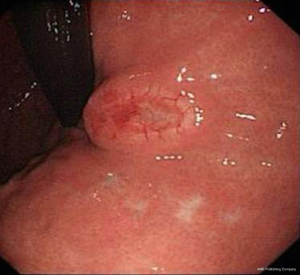
- Gastroscopy: gastroscopy revealed an irregular ridge-like lesion at the lesser curvature of the stomach body (about 39–40 cm away from the incisor tooth) (Figure 1);
- Gastroscopic pathology: the gastroscopic pathologic findings met the diagnosis standard of a well-differentiated NEN (G1), and immunohistochemical analysis showed CGA (+), SYN (+), and Ki-67<2%;
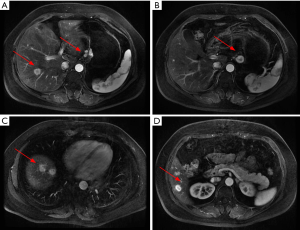
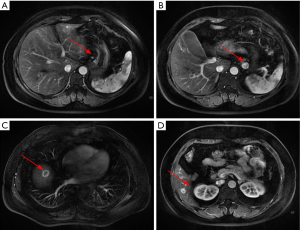
- Abdominal MRI: the multiple intrahepatic nodules with abnormal signals were considered to be multiple metastases. The wall of lesser curvature of the stomach was locally thickened. Multiple lymph node involvements were seen at left gastric flexure and were suspected to be metastases (Figure 2);
- Somatostatin receptor scintigraphy (SRS) in other hospital: multiple lesions with high somatostatin receptor expression were seen inside liver and near the lesser curvature of the stomach and were considered to be metastases. The wall of lesser curvature of the stomach was slightly thickened. No lesion with definite high somatostatin receptor expression was observed;
- Serum gastrin test in other hospital: 51.41 pg/mL (<100 pg/mL); and CgA 224.56 ng/mL (<100 ng/mL).
Diagnosis
- Gastric well-differentiated neuroendocrine neoplasm (NEN) (G1), type III, with perigastric lymph node metastasis;
- Multiple liver metastases.
Preoperative treatment
During the first multidisciplinary treatment (MDT) consultation, sandostatin treatment was recommended.
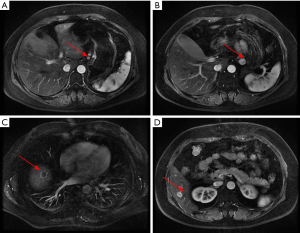
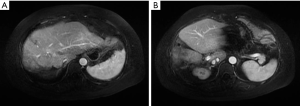
The patient received eight cycles of sandostatin treatment (20 mg, im) from November 24, 2014 to June 10, 2015. After two cycles of sandostatin treatment, abdominal MRI (Figure 3) during a follow-up examination showed that the intrahepatic metastases and perigastric lymph nodes were smaller than before, and the thickening of the wall of lesser curvature of the stomach was milder than before. Gastroscopy showed that the lesion in the lesser curvature was not remarkably changed. The overall efficacy was assessed as partial response (PR). After six cycles of sandostatin treatment, further abdominal MRI (Figure 4) and gastroscopy (Figure 5) revealed that the intrahepatic metastases, perigastric lymph node involvements, and lesion in the lesser curvature were basically same as before, and the overall efficacy was assessed as stable disease (SD).
During the second MDT consultation, surgical treatment was recommended to remove the primary gastric lesion and lower the tumor burden.
Surgical treatment
Proximal subtotal gastrectomy + right hepatectomy + left partial hepatectomy + cholecystectomy were performed under general anesthesia on July 1, 2015.
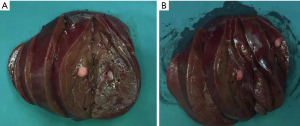
Intraoperative exploration showed that the primary lesion (sized about 2 cm × 3 cm) was located at the lesser curvature of the stomach body. Several swollen lymph nodes (sized about 0.5–2 cm) were palpable in the perigastric area. Intraoperative ultrasound showed multiple metastatic lesions in the right lobe and multiple lesions in left lobe; however, the lesions in the left lobe of the liver were mainly located on the liver surface, with just one lesion located deep inside the liver. Since a complete (R0) resection of the liver metastases was expected, proximal subtotal gastrectomy + right hepatectomy + left partial hepatectomy were then performed. After the surgery, intraoperative ultrasound was performed again to confirm that there was no residual tumor in liver. The gross appearance of metastases in the right lobe of liver was showed as follow (Figure 6).
Post-operative pathology
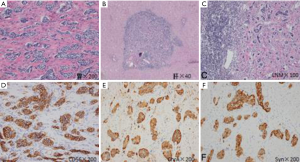
- (After proximal subtotal gastrectomy) gastric NEN (G1). No definite mitotic figure was seen. More intravascular cancer embolus and perineural invasion were observed. There was no obvious sign of tumor cell degeneration. The treatment response was poor. Tumor penetrated into the muscular layer of subserosal adipose tissue but did not involve the gastroesophageal junction and omentum (Figure 7A);
- (Right liver), (station 4a, 1 nodule), (station 4a, 3 nodules), (station 3, 2 nodules), (station 3, 1 nodule), (station 4b, nodules); intrahepatic multiple metastatic neuroendocrine tumors (NET); combined with Ki-67 labeling index (3%), the grade was G2; mitostic rate <1/10 HPF. The degeneration of tumor cells was obvious, accompanied with marked fibrosis, which met the diagnostic criteria of moderate post-operative change (Figure 7B);
- Metastases to lymph nodes (3/20), with local involvement seen outside the lymph node capsule (Figure 7C);
- pTNM staging: pT3N2M1;
- Immunohistochemical findings: stomach: AE1/AE3 (3+), CD56 (3+), CgA (2+), Syn (3+), Ki-67 (+1%), and SSTR2 (3+); liver metastasis: AE1/AE3 (3+), CD56 (3+), CgA (3+), Syn (3+), Ki-67 (+3%), and SSTR2 (3+) (Figure 7D-F).
Postoperative treatment and follow-up
Postoperative sandostatin treatment was recommended.
Abdominal MRI was performed on August 10, 2015. The stomach and liver showed postoperative changes, without obvious abnormality (Figure 8).
Discussion
Clinical classification of gNENs
Clinically, gNENs can be divided into well-differentiated gNENs (including types I, II, and III) and poorly-differentiated gNENs (i.e., type IV) according to the degree of gNENs tissue differentiation. The features of all gNEN types are as follows:
- Type I: type I is most common, accounting for about 70–80%. It is mainly related to type A atrophic gastritis, featured by elevated serum gastrin level and low gastric acid. The tumors are often multiple, with small diameters. Histopathologically, they are often well-differentiated NETs (G1). Type I gNENs tend to have a prognosis and rare distant metastasis;
- Type II: type II gNENs account for about 5–6%. While their pathogenesis is mainly associated with gastrinoma or MEN1, they can be associated with elevated serum gastrin level and high gastric acid. The tumors are often multiple, with small diameters. Histopathologically, they are often NET (G1) or NET (G2). Their prognoses are often poorer than type I gNENs. About 10–30% of type II gNENs already have distant metastasis when the disease is confirmed;
- Type III: type III gNENs account for about 14–25%. The patients have no atrophic gastritis or high gastrin-related diseases, in which the serum gastrin and gastric acid levels are normal. The tumors are often solitary, sized >2 cm. Histopathologically, they are often well-differentiated NETs (G1/G2/G3). More than 50% of newly diagnosed type III NET patients already have distant metastasis. The tumor-related case-fatality rate of type III NET is about 25–30%, and its prognosis is better than those of type I and type II gNENs;
- Type IV: type IV, namely NEC and MANEC, is a clinically rare type, featured by normal or slightly elevated blood gastrin level. The tumors are often solitary and the diameters of the type III lesions are often >5 cm. Patients with type IV gNENs have the poorest prognosis. Metastasis occurs in up to 80–100% of type IV gNEN patients, who tend to have short survival, with a tumor-related case-fatality rate of >50%.
Clinical features of gNENs
Gastroenteropancreatic neuroendocrine tumors (GEP-NETs) are a group of rare tumors with invasion and distant metastasis potentials that are composed of cells that have different morphologies but can secrete neuroendocrine hormones. Due to their insidious onset and non-specific symptoms, up to 40–95% of patients already have developed distant metastasis at the initial time of diagnosis (1). The liver is second only to regional lymph nodes as a site of metastasis. About 28.3–77.0% of pancreatic NET and 67–91% of small intestine NET are already accompanies with liver metastasis at the initial time of diagnosis (2-3); in contrast, primary NETs in stomach, appendix, and rectum with liver metastasis are rare (4). For NENs, liver metastasis is one of the most important diagnostic predictors (in addition to tumor grade) (3-5).
Since the further management of GEP-NET highly relies on the distribution of liver metastases, we divided the liver metastases into three types based on preoperative imaging findings (2):
- Type I: for liver metastases localized in one lobe or two adjacent segments, standard anatomical resection can achieve the en bloc resection of the lesion. Such “simple” cases of GEP-NET with liver metastasis account for about 20–25%;
- Type II: the metastatic lesions are mainly distributed in the right or left lobe of the liver; however, small satellites still exist in another lobe. This type accounts for about 10–15%. Clinically this type is mainly treated with multidisciplinary strategies including surgical resection (the mainstream treatment) and ablation therapy;
- Type III: the metastases are diffusely distributed in the left and right lobes of the liver and cannot be treated with surgery. This type accounts for about 60–70%.
Surgical indications of GEP-NET with liver metastasis
At present, surgical resection remains the mainstream treatment for GEP-NET with liver metastasis and also the only approach that is possible to achieve radical treatment. In patients with NET of midgut and hindgut origin with liver metastasis, the 5-year survival rate could reach 60–80% following the complete resection (R0/R1) of the primary lesions and metastases; in contrast, the 5-year survival rate was only 30% in patients who have not received surgical treatment (2).
Generally, the surgical treatment of GEP-NET with liver metastasis must meet the following conditions: (i) with resectable well-differentiated (G1/G2) liver metastases and the predicted peri-operative mortality rate was <5%; (ii) without right cardiac dysfunction; (iii) without unresectable lymph node metastasis or extraperitoneal metastases; and (iv) without diffuse or unresectable peritoneal metastasis. Typically, most patients have already developed type III hepatic metastases and thus lost the chance for surgery at the initial time of diagnosis; only 20–30% of patients may still have the chance for surgical treatment (6). In our current case, the patient already had gNEN (G1) with multiple liver metastases at first presentation. Based on preoperative imaging and intraoperative exploration findings, type II liver metastases were considered. Also, there was still a possible chance for radical resection. Right hepatectomy + resection of metastases in left liver were performed, with a residual liver volume of >30%. Thus, active treatment in a scientific manner may help to improve the life quality and prolong the survival in these patients. Surgery is often not recommended for patients with G3 NENs with liver metastasis; however, surgical resection may be considered for NEC with solitary resectable metastasis (2).
Summary
NENs are a group of rare tumors. For such a highly heterogeneous disease, an MDT team with specialists from the departments of surgical oncology, medical oncology, imaging, and molecular pathology is required to provide the patients with the optimal treatment protocol. Meanwhile, the MDT strategies including surgical treatment (the mainstream approach), local treatment (such as ablation, intervention, etc.) of liver lesions, somatostatin analogues, immunotherapy, chemotherapy, molecularly targeted therapy, and liver transplantation should be further applied in NENs management.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Frilling A, Modlin IM, Kidd M, et al. Recommendations for management of patients with neuroendocrine liver metastases. Lancet Oncol 2014;15:e8-21. [Crossref] [PubMed]
- Pavel M, Baudin E, Couvelard A, et al. ENETS Consensus Guidelines for the management of patients with liver and other distant metastases from neuroendocrine neoplasms of foregut, midgut, hindgut, and unknown primary. Neuroendocrinology 2012;95:157-76. [Crossref] [PubMed]
- Panzuto F, Nasoni S, Falconi M, et al. Prognostic factors and survival in endocrine tumor patients: comparison between gastrointestinal and pancreatic localization. Endocr Relat Cancer 2005;12:1083-92. [Crossref] [PubMed]
- Ahmed A, Turner G, King B, et al. Midgut neuroendocrine tumours with liver metastases: results of the UKINETS study. Endocr Relat Cancer 2009;16:885-94. [Crossref] [PubMed]
- Pape UF, Berndt U, Müller-Nordhorn J, et al. Prognostic factors of long-term outcome in gastroenteropancreatic neuroendocrine tumours. Endocr Relat Cancer 2008;15:1083-97. [Crossref] [PubMed]
- Frilling A, Clift AK. Therapeutic strategies for neuroendocrine liver metastases. Cancer 2015;121:1172-86. [Crossref] [PubMed]
Cite this article as: Chen X, Zhao H. Gastric neuroendocrine neoplasm with liver metastases: a case report. Transl Gastroenterol Hepatol 2016;1:55.