Conversion therapy for stage IV gastric cancer—the present and future
The problems we are facing and the definition of conversion therapy
Gastric cancer (GC) is the sixth most common form of cancer and the fourth most common form of cancer-related death in spite of efforts that have increased the frequency of its early diagnosis and improved intensive treatments (1). The incidence rate of gastric cancer in East Asian countries such as Japan, Korea and China is higher than that in Western countries. Chemotherapy is applied as the first treatment for metastatic and recurrent gastric cancer, even in cases that include a new treatment with a targeted therapy for HER2. The overall survival time remains at 16 months (2-6). In order to further improve the survival of stage IV gastric cancer patients, new therapeutic approaches should be considered (7,8). Conversion therapy for gastric cancer is one of the topics in this issue and successful treatment results have been reported (9-15). Although conversion therapy looks promising, it has not been scientifically proven due to its complicated nature and the fact that the number of candidate patients is too small to carry out a randomized control trial (RCT). Moreover, the following issues remain to be clarified: (I) what is the definition of conversion therapy? (II) What is the indication for the operation (liver, LN, peritoneal, or distant metastasis)? (III) What is the best chemotherapy regimen? (IV) What is the best timing of the operation (when the patient has stable disease or when it is regrowing)? (V) Is postoperative chemotherapy necessary? (VI) Is an R0 operation necessary?
We have previously defined conversion therapy for stage IV gastric cancer as a surgical treatment that aimed at achieving an R0 resection, when metastases that were unresectable or marginally resectable (for technical and/or oncological reasons) were controllable by chemotherapy. However, as mentioned above, the indications and procedure of the operation are associated with a great deal of confusion. Stage IV represents a complicated situation that involves peritoneal dissemination, hematological metastasis and distant lymph node metastasis. Considering this situation, we have recently demonstrated new categories of stage IV gastric cancer, which are defined based on oncosurgical treatment strategies (16).
The proposal of new categories of classification for stage IV GC
In order to better understand the biology and indications of curative surgery as a conversion therapy, we have proposed new categories (which have been demonstrated elsewhere) for the classification of stage IV gastric cancer. Some of the modifications are presented in Figure 1.
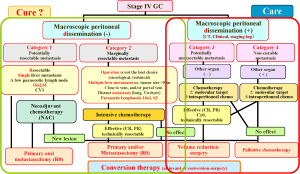
In our previous report, we stated (16):
“Stage IV GC patients can be divided based on the absence (categories 1 and 2) or the presence (categories 3 and 4) of macroscopic peritoneal dissemination. Category 1: potentially resectable metastasis; Category 2: marginally resectable metastasis; Category 3: incurable and unresectable except in cases in which local palliation is required; Category 4: non-curable metastasis. The indications for conversion therapy include category 2 patients, some category 3 patients and a very small number of category 4 patients in whom an R0 resection can be expected after a satisfactory response to chemotherapy (9,10).” Physicians may be able to aim at a ‘cure’ for some patients in categories 1 and 2; however, ‘care’ is the main goal of the treatment strategies for categories 3 or 4.
What we have learned from the REGATTA trial (17)
The REGATTA trial is a phase III study to investigate the survival benefit of gastrectomy + chemotherapy vs chemotherapy for patients with stage IV gastric cancer with a single incurable factor (either hepatic metastasis, peritoneal metastasis or para-aortic lymph node metastasis) confirmed by enhanced abdominal CT, an exploratory laparoscopic examination or laparotomy. Interestingly, the survival of patients who underwent palliative gastrectomy was not better than those who were treated without it, demonstrating that palliative gastrectomy is not beneficial and conversion therapy can therefore be considered as a potential next-generation strategy for stage IV GC.
Representative patients for each category
In this session, the case presentation and clinical questions for each category will be discussed.
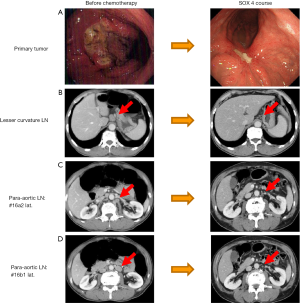
- Category 1: potentially resectable metastasis. This category includes patients with a single liver metastasis, with positive cytology or metastasis of the number 16a2 and/or 16b1 para-aortic LNs. Oncologically, this category is regarded as stage IV with technically resectable metastasis. Figure 2 demonstrates the case of 64-year-old male patient who was diagnosed with type 3 gastric cancer arising in the body of the stomach with distant metastasis LN numbers 16a2 and 16b1. Routine examinations revealed no obvious distant metastasis, liver metastasis or peritoneal dissemination;
- The diagnosis before treatment: This case is of course, regarded as stage IV [T4, N2, P0, H0, M1 (LYM)] but the primary tumor and the number 16a2 and 16b1 LNs may be technically resectable. Oncologically, however, the results of the REGATTA trial suggest that long survival cannot generally be expected (17). Chemotherapy was selected as the initial treatment;
- Chemotherapy selection: four courses of 4 SOX therapy were conducted. S-1 plus cisplatin (S-1/CDDP) is recommended as the first line chemotherapy in HER 2-negative patients, as well as S-1 plus docetaxel (DS) or capecitabine plus cisplatin (XP) (18) in the updated Japanese guidelines on gastric cancer (19). Moreover, oxaliplatin, which can be administered without hydration, has been used to replace cisplatin, because of the results of the G-SOX trial, which demonstrated the equal efficacy of the SOX regimen to the S-1/CDDP regimen with lower toxicity. Four cycles of SOX was therefore selected, which is the median number of treatment cycles that are administered with this regimen (20-22);
- Operation: total gastrectomy with D2 and para-aortic lymphadenectomy was performed. The pathological findings revealed a well-differentiated adenocarcinoma with invasion into the muscle layer (mp) with no microscopic lymph node metastasis detected among 78 retrieved lymph nodes or microscopic peritoneal dissemination;
- Postoperative chemotherapy: S-1 monotherapy was administered for 1 year;
- Prognosis: the patient remains alive without recurrence at 11 months without recurrence following the initial treatment (7 months after the operation). There should be a discussion on how distant LN dissection should be performed. In the this case, the para-aortic LN was regarded as technically resectable and therefore para-aortic LN dissection was performed, this included lymph node numbers 16a2, 16b1 and the sampling of 16a1 and 16b2 as well as D2 dissection (23-25). Although the case was regarded as stage IV, it was successfully down-staged to ypstage II by chemotherapy and S-1 monotherapy was administered for 1 year. Of course, there can be a discussion as to whether or not the SOX regimen should be conducted after a curative operation; theoretically, it should, but thus far there is no consensus;
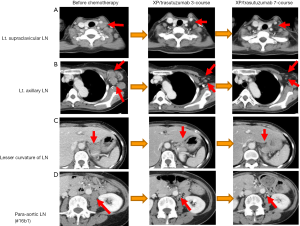
- Category 2: marginally resectable metastasis. This category include patients for whom an operation would not be considered to be the best choice for the initial treatment due to the presence of metastatic regions that are regarded as oncologically or technically unresectable. With regard to liver metastasis, patients with more than two liver metastases, a tumor of >5 cm in size or one that is located close to the hepatic and/or portal vein might be included in this category. As for LN metastasis, patients that have para-aortic LN (No. 16a1, 16b2) and distant LN metastasis, including the mediastinal, supraclavicular and axillary lymph nodes and distant organ metastasis might also be included in this category. Case 2 is demonstrated in Figure 3;
- The diagnosis before treatment: this case is a 62-year-old female patient with left axillar LN swelling who was diagnosed with type 4 gastric cancer (T3) arising in the lesser curvature of the stomach body with perigastric LN, para-aortic LN, left supraclavicular LN and left axillary LN swelling, without apparent peritoneal dissemination or other distant organ metastasis. The patient was diagnosed with stage IV GC with a HER2-positive primary tumor;
- Chemotherapy before the operation: seven cycles of XP/trastuzumab therapy. As the patient in this case was HER2-positive, trastuzumab therapy was administered as the standard therapy in combination with XP. The patient had distant metastases; thus, an operation was not the best choice and palliative chemotherapy was selected as the first choice of treatment. Interestingly, after one cycle of the treatment, a PR (according to the RECIST criteria) was observed—this was confirmed 1 month later. Moreover, after seven cycles of treatment, the supraclavicular, axillar and para-aortic LNs were observed to have completely shrunk and a CR was confirmed; however, the primary lesion of the tumor was still present;
- We decided to perform total gastrectomy with D2 lymphadenectomy. The pathological findings demonstrated a poorly differentiated adenocarcinoma, the invasion of which was restricted to the submucosal layer (sm1) and ypN3a (7/38) with a grade 2 chemotherapeutic effect;
- Postoperative chemotherapy was administered with a single cycle of XP/trastuzumab and the patient remains alive at more than 4 years after the initial treatment. In this case, there can be a discussion as to whether or not conversion surgery or adjuvant surgery should have been performed because even primary lesions may disappear with the continuation of chemotherapy. However, we considered that the tumor might eventually acquire resistance to chemotherapy or that adverse effects would appear after more cycles of the treatment and primary tumor resection should be performed while the tumor remained stable without new lesions. With regard to the postoperative treatment, life-long chemotherapy should have been performed; however, the treatment was abandoned due to an adverse event;
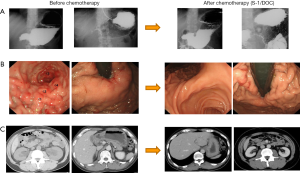
- Category 3: incurable and unresectable except when local palliation is necessary. This category includes patients in whom only peritoneal dissemination is detected by routine clinical examinations (as listed above), staging laparoscopy or at the time of the initial open laparotomy. A very interesting case is presented in Figure 4 as case 3. A 44-year-old male patient had gastric cancer arising from the body of the stomach which extended to the whole stomach (type 4) with pylorus stenosis, bilateral hydronephrosis due to peritoneal dissemination and obstructive jaundice was present. The biology might be described as so-called “retroperitoneal carcinomatosis”;
- The diagnosis before treatment: T3, Nx, P1 stage IV;
- Chemotherapy before the operation: S-1/DOC therapy was selected as the patient had bilateral hydronephrosis, thus platinum was not considered to be a good choice. After six cycles of the treatment, the patient’s hydronephrosis and obstructive jaundice were completely relieved; however, the primary tumor was still present;
- As there appeared to be no other lesions and the metastatic lesions remained stable, we attempted total gastrectomy with D2 lymphadenectomy. Fortunately, there was no obvious peritoneal dissemination in the abdominal cavity. The pathological findings demonstrated a well-differentiated adenocarcinoma, the invasion of which was restricted to the submucosal layer (sm1) and ypN0 (0/24) with a grade 2 chemotherapeutic effect;
- Theoretically, the same chemotherapy should be continued after conversion surgery; however, the patient refused doublet therapy and S-1 monotherapy was administered for 2 years after the operation. The patient remains alive at 96 months after surgery without recurrence and with no chemotherapy. In this category, when the metastatic site shows a good response to chemotherapy, the primary tumors and/or metastatic tumors can be removed after staging laparoscopy confirms CY0 and P0. Of course, one controversial opinion would be that palliative chemotherapy alone should have been continued fo the rest of the patient’s life; however, primary tumor resection was considered to be quite feasible because of the CR of the metastatic lesion and presence of the primary lesion. These operations can be defined as cytoreductive surgery or volume reduction surgery, even if a complete resection is performed because recurrence later develops in most of these cases (26). Of course, volume reduction surgery can be partly included in the definition of conversion therapy as a conversion surgery or adjuvant surgery. However, its clinical benefit should be clarified in the future.
Future perspectives
Controversy still exists among medical and/or surgical oncologists as to whether or not surgical intervention should be performed for patients with stage IV GC. According to the results of the REGATTA trial (17), palliative gastrectomy was not beneficial in cases other than those that involved bleeding or obstruction by the primary tumor. This result is very important because conversion therapy can be regarded as the next strategy for stage IV GC.
On the other hand, whether or not conversion therapy is beneficial remains controversial because the indications for the operation have not been clarified and because stage IV GC is composed of technically resectable metastasis and a tumor burden that is generally greater, a mixture of peritoneal dissemination, hematological metastasis and/or distant lymph node metastasis. For example, some physicians may be of the opinion that technically resectable metastasis can be regarded as conversion therapy; while others disagree because it is resectable, even stage IV and may aim to achieve a cure with a radical operation. Other physicians may consider the possible existence of numerous metastatic sites and thus never consider performing surgery with a curative intent.
Considering the lengthy discussions that have taken place, we have proposed new categories for classifying the biology of GC in order to understand the complicated situation of stage IV GC (16).
As described in previous reports, we have divided stage IV GC into two entities with macroscopic positive and negative patients, who are further classified into four categories (16). Patients without macroscopic peritoneal dissemination are classified into category 1 and category 2. The patients with potentially resectable metastasis are classified into category 1, while those with marginally resectable metastasis are classified into category 2. Patients with macroscopic peritoneal dissemination are classified into categories 3 and category 4. The patients in category 3 are considered to be incurable and have unresectable metastases; however, resection may be performed to achieve local palliation. The patients of category 4 have non-curable metastases. It is essentially impossible to a achieve cure in any patient with peritoneal carcinomatosis from gastric cancer, irrespective of the pretreatment extent or the ability to achieve an R0 resection. However, the survival outcomes differ according to the degree of disease progression and the extent of the disease, in addition to response to therapy. Longer survival can be expected in patients in categories 1, 2 who are treated with curative intent, while the treatment of other patients focuses on “care.” The concept of conversion therapy or adjuvant surgery principally includes patients in category 2, some patients in category 3 and rarely patients in category 4 when the operations are performed with the goal of achieving an R0 resection or a surgical cure.
Conversion therapy in patients with stage IV GC looks to be beneficial; however, it should be scientifically evaluated in an RCT or a prospective cohort study. A retrospective cohort study is now being conducted in Asia through the Federation of Asian Clinical Oncology (FACO), which consists of the Japanese Society of Clinical Oncology (JSCO), the Korean Association of Clinical Oncology (KACO) and the Chinese Society of Clinical Oncology (CSCO), with the support of the Japanese Gastric Cancer Association (JGCA), the Korean Gastric Cancer Association (KGCA) and the Gastric Cancer Association of the Chinese Anti-cancer Association. The primary endpoint is the incidence of operative complications and the secondary endpoints are OS and RFS according to the above-mentioned categories. The eligibility criteria include: (I) histologically proven primary gastric adenocarcinoma; (II) stage IV GC patients who are initially regarded as non-curably resectable but who are regarded as curably resectable (R0) and undergo radical surgery (including metastasectomy) after successful chemotherapy (categories 2, 3 and 4); (III) stage IV GC patients who are initially regarded as resectable, who undergo radical surgery after chemotherapy (neoadjuvant cases) (category 1); (IV) stage IV gastric cancer patients in whom peritoneal dissemination or positive cytology are detected by staging laparoscopy or laparotomy but who undergo radical surgery after successful chemotherapy, or patients who undergo gastrectomy irrespective of whether they receive chemotherapy (categories 1, 3). A further analysis will be required to clarify the benefits of conversion therapy in the new strategic approach for stage IV GC.
Acknowledgements
The authors acknowledge Ms. Kumi Mori, Aki Iwata, Kaori Enya and Kimiko Takano for their assistance with the preparation of the manuscript. This work was supported in part by Grants-in-Aid from the Japan Society of the Promotion of Science and from the Ministry of Health, Labour and Welfare of Japan.
Footnote
Conflicts of Interest: Kazuhiro Yoshida has received honoraria for lecture from Chugai Pharmaceutical Co., Ltd., Taiho Pharmaceutical Co., Ltd., Takeda Pharmaceutical Co., Ltd., Eli Lilly and Company, Daiichi Sankyo Co., Ltd., Ono Pharmaceutical Co., Ltd., Merck Serono Co., Ltd., Novartis Pharma K.K., Sanofi K.K.; and research funding from Ajinomoto Pharmaceutical Co., Ltd., Takeda Pharmaceutical Co., Ltd., Chugai Pharmaceutical Co., Ltd., Daiichi Sankyo Co., Ltd., Taiho Pharmaceutical Co., Ono Pharmaceutical Co., Yakult Honsha Co., Ltd., outside the submitted work. The other authors have no conflicts of interest to declare.
References
- GLOBOCAN 2012 database. GLOBOCAN database. Available online: http://globocan.iarc.fr/Default.aspx
- Koizumi W, Narahara H, Hara T, et al. S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer (SPIRITS trial): a phase III trial. Lancet Oncol 2008;9:215-21. [Crossref] [PubMed]
- Yoshida K, Ninomiya M, Takakura N, et al. Phase II study of docetaxel and S-1 combination therapy for advanced or recurrent gastric cancer. Clin Cancer Res 2006;12:3402-7. [Crossref] [PubMed]
- Koizumi W, Kim YH, Fujii M, et al. Addition of docetaxel to S-1 without platinum prolongs survival of patients with advanced gastric cancer: a randomized study (START). J Cancer Res Clin Oncol 2014;140:319-28. [Crossref] [PubMed]
- Tanabe K, Suzuki T, Tokumoto N, et al. Combination therapy with docetaxel and S-1 as a first-line treatment in patients with advanced or recurrent gastric cancer: a retrospective analysis. World J Surg Oncol 2010;8:40. [Crossref] [PubMed]
- Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 2010;376:687-97. [Crossref] [PubMed]
- Shen L, Shan YS, Hu HM, et al. Management of gastric cancer in Asia: resource-stratified guidelines. Lancet Oncol 2013;14:e535-47. [Crossref] [PubMed]
- Lordick F, Siewert JR. Recent advances in multimodal treatment for gastric cancer: a review. Gastric Cancer 2005;8:78-85. [Crossref] [PubMed]
- Yoshida K, Yamaguchi K, Okumura N, et al. The roles of surgical oncologists in the new era: minimally invasive surgery for early gastric cancer and adjuvant surgery for metastatic gastric cancer. Pathobiology 2011;78:343-52. [Crossref] [PubMed]
- Suzuki T, Tanabe K, Taomoto J, et al. Preliminary trial of adjuvant surgery for advanced gastric cancer. Oncol Lett 2010;1:743-747. [PubMed]
- Satoh S, Okabe H, Teramukai S, et al. Phase II trial of combined treatment consisting of preoperative S-1 plus cisplatin followed by gastrectomy and postoperative S-1 for stage IV gastric cancer. Gastric Cancer 2012;15:61-9. [Crossref] [PubMed]
- Han DS, Suh YS, Kong SH, et al. Outcomes of surgery aiming at curative resection in good responder to induction chemotherapy for gastric cancer with distant metastases. J Surg Oncol 2013;107:511-6. [Crossref] [PubMed]
- Okabe H, Ueda S, Obama K, et al. Induction chemotherapy with S-1 plus cisplatin followed by surgery for treatment of gastric cancer with peritoneal dissemination. Ann Surg Oncol 2009;16:3227-36. [Crossref] [PubMed]
- Satoh S, Hasegawa S, Ozaki N, et al. Retrospective analysis of 45 consecutive patients with advanced gastric cancer treated with neoadjuvant chemotherapy using an S-1/CDDP combination. Gastric Cancer 2006;9:129-35. [Crossref] [PubMed]
- Katayama H, Ito S, Sano T, et al. A Phase II study of systemic chemotherapy with docetaxel, cisplatin, and S-1 (DCS) followed by surgery in gastric cancer patients with extensive lymph node metastasis: Japan Clinical Oncology Group study JCOG1002. Jpn J Clin Oncol 2012;42:556-9. [Crossref] [PubMed]
- Yoshida K, Yamaguchi K, Okumura N, et al. Is conversion therapy possible in stage IV gastric cancer: the proposal of new biological categories of classification. Gastric Cancer 2016;19:329-38. [Crossref] [PubMed]
- Fujitani K, Yang HK, Mizusawa J, et al. Gastrectomy plus chemotherapy versus chemotherapy alone for advanced gastric cancer with a single non-curable factor (REGATTA): a phase 3, randomised controlled trial. Lancet Oncol 2016;17:309-18. [Crossref] [PubMed]
- Yamaguchi K, Sawaki A, Doi T, et al. Efficacy and safety of capecitabine plus cisplatin in Japanese patients with advanced or metastatic gastric cancer: subset analyses of the AVAGAST study and the ToGA study. Gastric Cancer 2013;16:175-82. [Crossref] [PubMed]
- Japanese Gastric Cancer Association. Japanese Guideline for Gastric Carcinoma. In: Sano T, editor. Kanehara-shuppan, 2014.
- Koizumi W, Takiuchi H, Yamada Y, et al. Phase II study of oxaliplatin plus S-1 as first-line treatment for advanced gastric cancer (G-SOX study). Ann Oncol 2010;21:1001-5. [Crossref] [PubMed]
- Hamada C, Yamada Y, Azuma M, et al. Meta-analysis supporting noninferiority of oxaliplatin plus S-1 to cisplatin plus S-1 in first-line treatment of advanced gastric cancer (G-SOX study): indirect comparison with S-1 alone. Int J Clin Oncol 2016. [Epub ahead of print]. [Crossref] [PubMed]
- Yamada Y, Higuchi K, Nishikawa K, et al. Phase III study comparing oxaliplatin plus S-1 with cisplatin plus S-1 in chemotherapy-naïve patients with advanced gastric cancer. Ann Oncol 2015;26:141-8. [Crossref] [PubMed]
- Kodera Y, Kobayashi D, Tanaka C, et al. Gastric adenocarcinoma with para-aortic lymph node metastasis: a borderline resectable cancer? Surg Today 2015;45:1082-90. [Crossref] [PubMed]
- Matsumoto T, Sasako M, Mizusawa J, et al. HER2 expression in locally advanced gastric cancer with extensive lymph node (bulky N2 or paraaortic) metastasis (JCOG1005-A trial). Gastric Cancer 2015;18:467-75. [Crossref] [PubMed]
- Tsuburaya A, Mizusawa J, Tanaka Y, et al. Neoadjuvant chemotherapy with S-1 and cisplatin followed by D2 gastrectomy with para-aortic lymph node dissection for gastric cancer with extensive lymph node metastasis. Br J Surg 2014;101:653-60. [Crossref] [PubMed]
- Rudloff U, Langan RC, Mullinax JE, et al. Impact of maximal cytoreductive surgery plus regional heated intraperitoneal chemotherapy (HIPEC) on outcome of patients with peritoneal carcinomatosis of gastric origin: results of the GYMSSA trial. J Surg Oncol 2014;110:275-84. [Crossref] [PubMed]
Cite this article as: Yamaguchi K, Yoshida K, Tanaka Y, Matsuhashi N, Tanahashi T, Takahashi T. Conversion therapy for stage IV gastric cancer—the present and future. Transl Gastroenterol Hepatol 2016;1:50.


