Ongoing surgical clinical trials on minimally invasive surgery for gastric cancer: Korea
Introduction
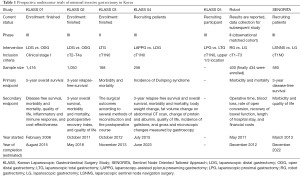
Despite the rapid progress in the molecular understanding of gastric cancer and the development of targeted therapies to treat it, currently surgical resection is the only effective treatment option to improve the survival (1-3). Since the first successful gastrectomy for gastric cancer by Theodor Billroth in 1881, the surgical skill has been steadily revising and improving. Remarkable change during recent decades would be the adoption of minimally invasive surgery (MIS) for gastric cancer. Due to the recent improvements in early diagnosis (4), the incidence of early gastric cancer (EGC) in Korea has been increasing and MIS has been rapidly adopted to many Korean gastric cancer surgeons. Moreover, the efforts to build the evidence on feasibility of MIS are being continued. As experience in the use of laparoscopy has accumulated, the inclusion criteria for studies on MIS have been extended to patients with advanced gastric cancer (AGC). Moreover, interests in advanced techniques, such as laparoscopic total gastrectomy (LTG) or extended lymph node dissection has steadily broadened the scope of the studies on MIS. In addition, the effort to find a more suitable surgical treatment option in EGC, such as sentinel node navigation surgery (SNNS) or function preserving surgery is also continued. The aim of this article is to overview the current status of ongoing clinical trials regarding MIS for gastric cancer in Korea (Table 1).
Full table
Laparoscopic gastrectomy for EGC
In Korea, the Korean Laparoscopic Gastrointestinal Surgery Study (KLASS)-01 trials—the first multicenter, large-scale, prospective, randomized controlled trial (RCT) comparing laparoscopic distal gastrectomy (LDG) with open distal gastrectomy (ODG) for EGC—was started in 2006 (NCT00452751). Although several RCTs had been conducted to address the oncologic safety of laparoscopic gastrectomy before commencing KLASS-01, the sample sizes of those studies were not large enough to conclusively demonstrate safety and equivalency compared with open procedures (5-8). The primary end point of the KLASS-01 was 5-year overall survival. The secondary endpoints were disease free survival, morbidity and mortality, quality of life, inflammatory, immune response, and cost-effectiveness. The Enrollment was finished at August 2010; 1,416 patients from 12 centers participated in this study. Recently, the result on morbidity and mortality was reported (9). The overall complication rate was significantly lower in the LDG group (13.0%) than in ODG group (19.9%) although there was no difference in interim analysis (10). The difference was mainly due to wound complication (LDG vs. ODG; 3.1% vs. 7.7%, P = 0.001). The result confirms the minimally invasiveness of laparoscopic surgery. The final results on oncologic safety are expected to be reported soon.
In spite of rapid progress on surgical skill and instruments, LTG in gastric cancer patients is not widely accepted due to the absence of established optimal methods for anastomosis and the technical difficulty of performing complete D2 lymphadenectomy. To investigate the feasibility of LTG in clinical stage I gastric cancer located in upper 1/3, the KLASS group launched the prospective, multicenter phase II trial (KLASS-03) in 2012. The purposes of this study are to evaluate the incidence of postoperative morbidity and mortality, and to evaluate the surgical outcomes according to several methods of reconstruction. Also, the postoperative course of the patients underwent LTG was analyzed. The enrollment of 168 patients was finished in November 2013. The result of this study will confirm the safety of LTG and propose the optimal method of anastomosis in LTG (NCT01584336).
Laparoscopic gastrectomy for AGC
The standard surgical treatment for AGC is open gastrectomy with D2 lymphadenectomy according to Japanese, NCCN, and ESMO guidelines (11-13). D2 lymphadenectomy is associated with lower loco-regional recurrence and gastric cancer-related death rates compared to gastrectomy with D1 lymphadenectomy (14). However, the actual extent of D2 lymphadenectomy varies among surgeons because of a lacking consensus on the anatomical definition of each lymph node station. It was a big obstacle to perform the RCT comparing laparoscopic and open D2 lymphadenectomy for patients with locally AGC. Therefore, standardization of D2 lymphadenectomy and surgical quality control (KLASS-02-QC, NCT01283893) were accomplished prior to KLASS-02-RCT trial (15) to build a consensus on D2 lymphadenectomy and to qualify surgeons. Six unedited videos of LDG and ODG were submitted by surgeons for participation and reviewed by international experts using evaluation criteria for completeness of D2 lymphadenectomy. Finally, the review committee made decisions on whether a surgeon’s qualification was sufficient to participate in KLASS-02-RCT (16). The primary endpoint of the KLASS-02 RCT is non-inferiority in the 3-year relapse-free survival rate after LDG and D2 lymphadenectomy for locally AGC compared with open conventional surgery. The secondary end-points are 3-year overall survival, morbidity and mortality, postoperative recovery index, and quality of life (NCT0146598). The estimated sample size of KLASS-02 is 1,050. The enrollment of patients was finished in May 2015 and the final results are expected to be reported in 2018. The KLASS-02 trial is the first phase III trial to evaluate the efficacy of LDG with D2 lymphadenectomy for AGC. Also in China (CLASS 01, NCT01609309) and Japan (JLSSG0901, UMIN000003420), a multicenter phase III trials are ongoing to compare LDG and ODG in patients with locally AGC.
Laparoscopic function-preserving surgery
The improved survival rates of cancer patients have increased the interest in patients’ post-surgical quality of life. The aim of function preserving surgery in gastric cancer patient is to reduce the functional sequelae of radical gastrectomy such as dumping syndrome, reflux gastro-esophagitis and weight loss. Function preserving gastric resections include pylorus-preserving gastrectomy (PPG), proximal gastrectomy (PG), and vagus nerve preserving gastrectomy. With the development of minimally invasive approaches, methods to adopt those function preserving procedures to MIS have been tried.
PPG has been known to have functional advantages in terms of nutritional benefit, lower incidence of dumping syndrome, bile reflux, or gallstone formation, as compared with distal gastrectomy (17-21). However, previous comparative studies between PPG and distal gastrectomy were mostly performed retrospectively by conventional open surgery. In addition, PPG may have potential risks against oncologic safety, including fewer dissected lymph nodes compared to distal gastrectomy (22-24). Although recent large-volume retrospective analyses reported that laparoscopic PPG was oncologically safe and was better than LDG in terms of nutritional advantage and a lower incidence of gallstone formation, prospective RCTs, especially comparing laparoscopic PPG and LDG, are rare (25). To evaluate superiority on postoperative quality of life and comparable survival after laparoscopic PPG compared to LDG in patients with middle-third EGC, KLASS-04 trial is recruiting patients since July 2015 (NCT02595086). The primary endpoint is incidence of dumping syndrome, and secondary endpoints are 3-year relapse-free survival and overall survival, morbidity and mortality, body weight change, fat volume change on abdominal CT scan, change of protein and albumin, quality of life, incidence of gallstone, and gross and microscopic changes measured by gastroscopy. The estimated sample size is 256. This study will contribute to the wide application of laparoscopic PPG.
Performing PG for gastric cancer has been limited because of anastomosis related complications such as anastomosis stricture and reflux esophagitis, which substantially affected postoperative quality of life after the surgery. Although many investigators reported the various types of reconstructions and feasibility of these methods, there was no RCT comparing laparoscopic PG with LTG (26-28). Recently, double tract reconstruction following PG was reported as feasible and useful method with excellent postoperative outcomes in terms of preventing reflux symptoms (4.65%) and anastomotic stenosis (4.65%) by the investigators in Korea (28). The KLASS group is preparing the KLASS-05 trial comparing Laparoscopic PG with double tract reconstruction and LTG. The primary endpoint is hemoglobin change at post-gastrectomy 2 years, and secondary endpoints are prevalence rate of postoperative reflux esophagitis, anastomotic stricture, incidence of morbidity and mortality, quality of life 2-year after operations and 3-year disease free survivals. The estimated sample size is 180. Currently, recruitment of participating surgeons is in progress.
Sentinel node navigation surgery (SNNS)
SNNS and function preserving surgery share the concept. SNNS, the individualized minimally invasive treatment, may retain the patients’ quality of life by preventing various post-gastrectomy syndromes related to unnecessary prophylactic lymph node dissection in patients without lymph node metastasis. However, clinical application of SNNS remains controversial for years because of different study protocol and results between studies (29-34). Main debatable issue for clinical use of SNNS is high false negative rate. However, a recent multicenter trial from Japan showed quite promising results with a low false-negative rate of SN biopsy in early-staged gastric cancer patients (35). In addition, multicenter quality control study (phase II) has been performed recently in Korea prior to phase III trial and tolerable results were observed (0% false negative rate of laparoscopic sentinel node basin dissection) (36). Based on these results, multicenter phase III trial (Sentinel Node Oriented Tailored Approach, SENORITA) to compare the laparoscopic SNNS with laparoscopic gastrectomy for cEGC (cT1N0, less than 3 cm, not indicated to endoscopic submucosal resection) was launched in March 2013 (NCT01804998). The estimated sample size was 580. The primary end-point was 3-year disease-free survival, and the secondary end-points were morbidity and quality of life. Laparoscopic SNNS is expected to assume an important role in gastric cancer treatment through SENORITA trial. However, there are a number of technical problems to be resolved for clinical use of SNNS. These include the accuracy of intraoperative pathological diagnosis, optimal tracer material or method, and the possible applicability of intraoperative endoscopic resection instead of partial gastric resection. Additional studies on SNNS are still required.
Robotic surgery for gastric cancer
Da Vinci robot systems (Intuitive Surgical, Sunnyvale, California, USA) was applied to gastric cancer in Korea since 2005 and several investigators have reported that short-term postoperative outcomes and oncologic outcomes of robot surgery were comparable to laparoscopic gastrectomy (37-40). In addition, meta-analysis results revealed that use of robotic surgery for gastric cancer significantly decreases intraoperative blood loss. Also, comparable morbidity and mortality to laparoscopic surgery was reported (41). However, longer operation time and higher cost are the limitations of clinical application of robotic surgery in gastric cancer in spite of technical superiority over laparoscopic surgery such as 3-dimensional imaging, surgical instrument with a high degree of angulation and filtration of resting tremor. The current indications for robotic surgery are similar to laparoscopic surgery due to a lack of evidence. Therefore, a prospective, multicenter comparative study was conducted in Korea and the short-term outcome was reported recently (NCT01309256). There were no significant differences in morbidity and mortality rates, estimated blood loss, rates of open conversion, diet build-up, and length of hospital stay, but significantly higher cost and longer operation time in robot group were observed as expected (42). Studies on the long-term surgical outcomes are on progress.
Conclusions
This article is a brief outline of ongoing clinical trials on MIS in Korea. Well-designed, large-scale clinical studies had been completed or are actively ongoing. The results of the studies are expected to prove that MIS is as safe and effective as open conventional surgery. As well as accumulation of evidence, the multicenter prospective studies have contributed to the standardization of the surgical technique. This has also accelerated the subsequent clinical trials. Many unresolved issues of MIS are expected to be addressed in future multicenter prospective studies.
Acknowledgements
None.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Van Ness M, Gregg J, Wang J, et al. Genetics and molecular pathology of gastric malignancy: Development of targeted therapies in the era of personalized medicine. J Gastrointest Oncol 2012;3:243-51. [PubMed]
- Dittmar Y, Settmacher U. Individualized treatment of gastric cancer: Impact of molecular biology and pathohistological features. World J Gastrointest Oncol 2015;7:292-302. [Crossref] [PubMed]
- Sokic-Milutinovic A, Alempijevic T, Milosavljevic T. Role of Helicobacter pylori infection in gastric carcinogenesis: Current knowledge and future directions. World J Gastroenterol 2015;21:11654-72. [Crossref] [PubMed]
- Hyung WJ, Kim SS, Choi WH, et al. Changes in treatment outcomes of gastric cancer surgery over 45 years at a single institution. Yonsei Med J 2008;49:409-15. [Crossref] [PubMed]
- Huscher CG, Mingoli A, Sgarzini G, et al. Laparoscopic versus open subtotal gastrectomy for distal gastric cancer: five-year results of a randomized prospective trial. Ann Surg 2005;241:232-7. [Crossref] [PubMed]
- Hayashi H, Ochiai T, Shimada H, et al. Prospective randomized study of open versus laparoscopy-assisted distal gastrectomy with extraperigastric lymph node dissection for early gastric cancer. Surg Endosc 2005;19:1172-6. [Crossref] [PubMed]
- Kitano S, Shiraishi N, Fujii K, et al. A randomized controlled trial comparing open vs laparoscopy-assisted distal gastrectomy for the treatment of early gastric cancer: an interim report. Surgery 2002;131:S306-11. [Crossref] [PubMed]
- Lee JH, Han HS, Lee JH. A prospective randomized study comparing open vs laparoscopy-assisted distal gastrectomy in early gastric cancer: early results. Surg Endosc 2005;19:168-73. [Crossref] [PubMed]
- Kim W, Kim HH, Han SU, et al. Decreased Morbidity of Laparoscopic Distal Gastrectomy Compared With Open Distal Gastrectomy for Stage I Gastric Cancer: Short-term Outcomes From a Multicenter Randomized Controlled Trial (KLASS-01). Ann Surg 2016;263:28-35. [Crossref] [PubMed]
- Kim HH, Hyung WJ, Cho GS, et al. Morbidity and mortality of laparoscopic gastrectomy versus open gastrectomy for gastric cancer: an interim report--a phase III multicenter, prospective, randomized Trial (KLASS Trial). Ann Surg 2010;251:417-20. [Crossref] [PubMed]
- Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer 2011;14:113-23. [Crossref] [PubMed]
- Ajani JA, Bentrem DJ, Besh S, et al. Gastric cancer, version 2.2013: featured updates to the NCCN Guidelines. J Natl Compr Canc Netw 2013;11:531-46. [PubMed]
- Okines A, Verheij M, Allum W, et al. Gastric cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2010;21 Suppl 5:v50-4. [Crossref] [PubMed]
- Songun I, Putter H, Kranenbarg EM, et al. Surgical treatment of gastric cancer: 15-year follow-up results of the randomised nationwide Dutch D1D2 trial. Lancet Oncol 2010;11:439-49. [Crossref] [PubMed]
- Kim HI, Hur H, Kim YN, et al. Standardization of D2 lymphadenectomy and surgical quality control (KLASS-02-QC): a prospective, observational, multicenter study BMC Cancer 2014;14:209. [NCT01283893]. [Crossref] [PubMed]
- Hur H, Lee HY, Lee HJ, et al. Efficacy of laparoscopic subtotal gastrectomy with D2 lymphadenectomy for locally advanced gastric cancer: the protocol of the KLASS-02 multicenter randomized controlled clinical trial. BMC Cancer 2015;15:355. [Crossref] [PubMed]
- Isozaki H, Okajima K, Momura E, et al. Postoperative evaluation of pylorus-preserving gastrectomy for early gastric cancer. Br J Surg 1996;83:266-9. [Crossref] [PubMed]
- Park DJ, Lee HJ, Jung HC, et al. Clinical outcome of pylorus-preserving gastrectomy in gastric cancer in comparison with conventional distal gastrectomy with Billroth I anastomosis. World J Surg 2008;32:1029-36. [Crossref] [PubMed]
- Hotta T, Taniguchi K, Kobayashi Y, et al. Postoperative evaluation of pylorus-preserving procedures compared with conventional distal gastrectomy for early gastric cancer. Surg Today 2001;31:774-9. [Crossref] [PubMed]
- Shibata C, Shiiba KI, Funayama Y, et al. Outcomes after pylorus-preserving gastrectomy for early gastric cancer: a prospective multicenter trial. World J Surg 2004;28:857-61. [Crossref] [PubMed]
- Nunobe S, Sasako M, Saka M, et al. Symptom evaluation of long-term postoperative outcomes after pylorus-preserving gastrectomy for early gastric cancer. Gastric Cancer 2007;10:167-72. [Crossref] [PubMed]
- Kong SH, Kim JW, Lee HJ, et al. The safety of the dissection of lymph node stations 5 and 6 in pylorus-preserving gastrectomy. Ann Surg Oncol 2009;16:3252-8. [Crossref] [PubMed]
- Hiki N, Shimoyama S, Yamaguchi H, et al. Laparoscopy-assisted pylorus-preserving gastrectomy with quality controlled lymph node dissection in gastric cancer operation. J Am Coll Surg 2006;203:162-9. [Crossref] [PubMed]
- Hiki N, Sano T, Fukunaga T, et al. Survival benefit of pylorus-preserving gastrectomy in early gastric cancer. J Am Coll Surg 2009;209:297-301. [Crossref] [PubMed]
- Suh YS, Han DS, Kong SH, et al. Laparoscopy-assisted pylorus-preserving gastrectomy is better than laparoscopy-assisted distal gastrectomy for middle-third early gastric cancer. Ann Surg 2014;259:485-93. [Crossref] [PubMed]
- Kinoshita T, Gotohda N, Kato Y, et al. Laparoscopic proximal gastrectomy with jejunal interposition for gastric cancer in the proximal third of the stomach: a retrospective comparison with open surgery. Surg Endosc 2013;27:146-53. [Crossref] [PubMed]
- Kim DJ, Lee JH, Kim W. Lower esophageal sphincter-preserving laparoscopy-assisted proximal gastrectomy in patients with early gastric cancer: a method for the prevention of reflux esophagitis. Gastric Cancer 2013;16:440-4. [Crossref] [PubMed]
- Ahn SH. Laparoscopic double-tract proximal gastrectomy for proximal early gastric cancer. Gastric Cancer 2014;17:562-70. [Crossref] [PubMed]
- Kitagawa Y, Fujii H, Mukai M, et al. Radio-guided sentinel node detection for gastric cancer. Br J Surg 2002;89:604-8. [Crossref] [PubMed]
- Hayashi H, Ochiai T, Mori M, et al. Sentinel lymph node mapping for gastric cancer using a dual procedure with dye- and gamma probe-guided techniques. J Am Coll Surg 2003;196:68-74. [Crossref] [PubMed]
- Kim MC, Kim HH, Jung GJ, et al. Lymphatic mapping and sentinel node biopsy using 99mTc tin colloid in gastric cancer. Ann Surg 2004;239:383-7. [Crossref] [PubMed]
- Tonouchi H, Mohri Y, Tanaka K, et al. Laparoscopic lymphatic mapping and sentinel node biopsies for early-stage gastric cancer: the cause of false negativity. World J Surg 2005;29:418-21. [Crossref] [PubMed]
- Park do J, Kim HH, Park YS, et al. Simultaneous indocyanine green and (99m)Tc-antimony sulfur colloid-guided laparoscopic sentinel basin dissection for gastric cancer. Ann Surg Oncol 2011;18:160-5. [Crossref] [PubMed]
- Miyashiro I, Hiratsuka M, Sasako M, et al. High false-negative proportion of intraoperative histological examination as a serious problem for clinical application of sentinel node biopsy for early gastric cancer: final results of the Japan Clinical Oncology Group multicenter trial JCOG0302. Gastric Cancer 2014;17:316-23. [Crossref] [PubMed]
- Kitagawa Y, Takeuchi H, Takagi Y, et al. Sentinel node mapping for gastric cancer: a prospective multicenter trial in Japan. J Clin Oncol 2013;31:3704-10. [Crossref] [PubMed]
- Lee YJ, Jeong SH, Hur H, et al. Prospective Multicenter Feasibility Study of Laparoscopic Sentinel Basin Dissection for Organ Preserving Surgery in Gastric Cancer: Quality Control Study for Surgical Standardization Prior to Phase III Trial. Medicine (Baltimore) 2015;94:e1894. [Crossref] [PubMed]
- Song J, Oh SJ, Kang WH, et al. Robot-assisted gastrectomy with lymph node dissection for gastric cancer: lessons learned from an initial 100 consecutive procedures. Ann Surg 2009;249:927-32. [Crossref] [PubMed]
- Hyun MH, Lee CH, Kwon YJ, et al. Robot versus laparoscopic gastrectomy for cancer by an experienced surgeon: comparisons of surgery, complications, and surgical stress. Ann Surg Oncol 2013;20:1258-65. [Crossref] [PubMed]
- Yoon HM, Kim YW, Lee JH, et al. Robot-assisted total gastrectomy is comparable with laparoscopically assisted total gastrectomy for early gastric cancer. Surg Endosc 2012;26:1377-81. [Crossref] [PubMed]
- Woo Y, Hyung WJ, Pak KH, et al. Robotic gastrectomy as an oncologically sound alternative to laparoscopic resections for the treatment of early-stage gastric cancers. Arch Surg 2011;146:1086-92. [Crossref] [PubMed]
- Xiong B, Ma L, Zhang C. Robotic versus laparoscopic gastrectomy for gastric cancer: a meta-analysis of short outcomes. Surg Oncol 2012;21:274-80. [Crossref] [PubMed]
- Kim HI, Han SU, Yang HK, et al. Multicenter Prospective Comparative Study of Robotic Versus Laparoscopic Gastrectomy for Gastric Adenocarcinoma. Ann Surg 2016;263:103-9. [Crossref] [PubMed]
Cite this article as: Lee JH. Ongoing surgical clinical trials on minimally invasive surgery for gastric cancer: Korea. Transl Gastroenterol Hepatol 2016;1:40.


