Laparoscopic splenic hilar lymphadenectomy for advanced gastric cancer
Introduction
Laparoscopic distal gastrectomy (LDG) has become widespread as a treatment of early gastric cancer in the distal stomach especially in Eastern Asia with the short-term advantages such as less blood loss and prompter postoperative recovery (1). LDG has recently been applied to advanced gastric cancer, and several large-scale randomized controlled trials comparing open and laparoscopic distal gastrectomy for advanced gastric cancer in the distal stomach have been performed in Korea and Japan (2,3) to evaluate feasibility and long-term oncologic outcome of LDG. However, the use of laparoscopic total gastrectomy (LTG) remains limited because of the high technical demands of esophagojejunostomy (4-6) and the complexity of lymphadenectomy at the splenic hilum (5,7,8-21). Because of the variation of the vascular anatomy in the splenic hilum and with the concern of pancreas-related complications, splenic hilar lymphadenectomy is technically challenging even for skilled surgeons. Based on the evidence that prophylactic combined resection of spleen in total gastrectomy increased the risk of postoperative morbidity (22,23) or had no survival benefit (24,25), surgeons have preferred laparoscopic spleen-preserving splenic hilar lymphadenectomy (LSPL) (8-19) rather than LTG with splenectomy (20,21). Since the first report by Hyung et al. (8) in 2008, the number of studies with acceptable feasibility of LSPL has increased (8-19). For further advanced cases, such as with metastasis to splenic hilar nodes or invasion to the greater curvature of the stomach, or with direct invasion to distal pancreas, LTG with splenectomy, sometimes with combined resection of distal pancreas has been performed (19-21). In this paper, the recent reports of LTG with splenic hilar lymphadenectomy were reviewed.
Inclusion/exclusion criteria for the review
For the review of laparoscopic splenic hilar lymphadenectomy, an English literature search was performed on the PubMed database using the terms ‘‘gastric cancer’’ AND ‘‘laparoscopic’’ AND ‘‘splenic hilar lymphadenectomy’’ along with their synonyms or abbreviations on December 23, 2015. Case series including less than 10 patients, or technical reports without surgical outcomes were excluded to keep the quality of the review. The endpoints were clinical indication, the length of the operation, blood loss, conversion, overall morbidity, mortality, length of the hospital stay, and number of harvested lymph nodes (in total and in the splenic hilum). As a result, 15 studies were included in this review. Tumor stage was classified according to the 7th edition of TNM classification (26). Postoperative complications were classified according to the Clavien-Dindo classification system (27).
Laparoscopic spleen-preserving splenic hilar lymphadenectomy (LSPL) (Table 1)
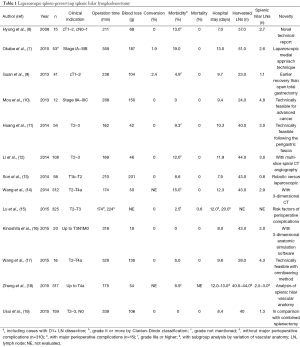
Full table
Since Hyung et al. (8) firstly reported the initial case series of LSPL with the acceptable feasibility, the number of patients included in the following studies has increased. Some technical reports provided better anatomical understandings. We have proposed efficient lymphadenectomy technique with ‘medial approach’ (5) by identifying the membranous border between the perigastric tissue and the surface of the retroperitoneum. The concept following the perigastric fascias and the intrafascial space based on embryological and anatomical background was also helpful (11). Together with the technical progress, comparative study of laparoscopy-assisted total gastrectomy (LATG) with LSPL and open total gastectomy for clinical T1-T2 tumors (9) was performed. Longer operation time, less blood loss, and earlier postoperative recovery were found in LATG with LSPL, which was consistent with the previous results of LDG (1). Gradually this operative procedure was applied to more advanced tumors (10-12,14-19), unless they had definite lymph node enlargement in the splenic hilum or direct tumor invasion of the gastrosplenic ligament. Among the 13 studies with LSPL, the indication was up to T2 in three studies, and up to T3 in five and T4a in five studies, respectively. The overall morbidity rate was 6–19%, which was acceptable, but Lu et al. (15) revealed in the study with 325 cases, that BMI exceeding 25 kg/m2, tumor location in the greater curvature, and No.10 LN metastases were significantly associated with increased rates of major perioperative complications, and further consideration of optimal indication seemed required. Because there are anatomical variations in the splenic hilum, preoperative evaluation by three-dimensional (3D) CT angiography was helpful to accomplish LSPL safely (12,14,16,18). Kinoshita et al. (16) used integrated 3D anatomic simulation software, which was also helpful in enhancing the quality of surgery. Robotic approach might be also helpful in completing technically-demanding LSPL procedure with current laparoscopic instruments (13).
Regarding the surgical outcomes of LSPL among the 13 studies, the operation time and blood loss ranged from 162 to 359 minutes, and 18 to 201 g, respectively. The length of hospital stay ranged from 7 to 13 days. The mortality rate was extremely low, and with the low overall morbidity rate (6–19%), LSPL seemed technically feasible with acceptable short-term surgical outcome.
LTG with splenectomy (Tables 2,3)

Full table

Full table
Because prophylactic combined resection of spleen increased the risk of postoperative morbidity (22,23) with no survival benefit (24,25) in open total gastrectomy, the reports on LTG with splenectomy were limited (19-21). There were only small case series so far. The indication was for advanced tumors such as T3-T4aN1-2 (19), or tumors invading the greater curvature of the upper third of the stomach, pancreatic parenchyma, or spleen (20), in which splenectomy was mandatory to accomplish R0 resection. These reports showed technical feasibility of this procedure, but the number of the patients included in the studies were limited. Further larger study is required for precise evaluation of this procedure.
Discussion
Splenic hilar lymphadenectomy should be employed in the treatment of advanced proximal gastric cancer to complete D2 dissection, and LTG with LSPL or splenectomy are selected. Because combined splenectomy increased the risk of postoperative morbidity and mortality in randomized clinical trials and could not show survival benefit compared with spleen preservation (22-25), routine or prophylactic splenectomy is not recommended by National Comprehensive Cancer Network guidelines (28). Recently, a large, multicenter, randomized controlled trial with 505 patients comparing splenectomy with spleen preservation on the proximal gastric cancer was performed (29,30). Proximal gastric adenocarcinoma of T2-4/N0-2/M0 not invading the greater curvature was eligible, and splenectomy resulted in higher morbidity, larger blood loss, and no survival advantage. The 5-year overall survivals were 75.1% and 76.4% in the splenectomy and spleen-preserving arms respectively, and the non-inferiority of spleen preservation was confirmed. They concluded that prophylactic splenectomy should be avoided not only for operative safety but also for survival benefit.
Even with the evidence described above, further advanced tumors such as those with direct invasion of the gastrosplenic ligament, pancreatic parenchyma, or spleen need to be resected by total gastrectomy with splenectomy, sometimes with combined resection of distal pancreas. Laparoscopic resection of such advanced tumors is technically demanding because huge tumor prevents laparoscopic view, or handling of the tumor is sometimes difficult, and care must be taken not to manipulate the tumor. Technical improvement for better short-term outcomes and validation of oncological outcomes with longer follow-up data would be required.
LTG with LSPL has gradually become popular with acceptable surgical outcomes, but careful interpretation is required. These excellent surgical results were provided by laparoscopic expert surgeons. Even if prophylactic splenectomy was denied, D2 lymphadenectomy for advanced gastric cancer is still a standard (31) for advanced gastric cancer. LTG with LSPL is still technically difficult for many surgeons and cannot be a standard at this moment. Further technical progress or acceptance of more simplified concept of lymphadenectomy, such as ‘D2-No.10’ lymphadenectomy for some limited cases might be required for LTG to be a first choice for advanced gastric cancer.
Conclusions
With the short-term advantage over open gastrectomy, laparoscopic gastrectomy has been applied not only in early but also advanced gastric cancer, or more complicated procedures such as LTG with LSPL or splenectomy. With the development of laparoscopic devices, advanced knowledge of laparoscopic view, and accumulated technical experiences, such laparoscopic advanced surgery could be feasible in near future. And by overcoming a critical validation of oncological outcomes, it still has a chance to be a procedure of choice as a treatment for advanced gastric cancer.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Viñuela EF, Gonen M, Brennan MF, et al. Laparoscopic versus open distal gastrectomy for gastric cancer: a meta-analysis of randomized controlled trials and high-quality nonrandomized studies. Ann Surg 2012;255:446-56. [Crossref] [PubMed]
- Hur H, Lee HY, Lee HJ, et al. Efficacy of laparoscopic subtotal gastrectomy with D2 lymphadenectomy for locally advanced gastric cancer: the protocol of the KLASS-02 multicenter randomized controlled clinical trial. BMC Cancer 2015;15:355. [Crossref] [PubMed]
- Inaki N, Etoh T, Ohyama T, et al. A Multi-institutional, Prospective, Phase II Feasibility Study of Laparoscopy-Assisted Distal Gastrectomy with D2 Lymph Node Dissection for Locally Advanced Gastric Cancer (JLSSG0901). World J Surg 2015;39:2734-41. [Crossref] [PubMed]
- Tsunoda S, Okabe H, Obama K, et al. Short-term outcomes of totally laparoscopic total gastrectomy: experience with the first consecutive 112 cases. World J Surg 2014;38:2662-7. [Crossref] [PubMed]
- Okabe H, Tsunoda S, Tanaka E, et al. Is laparoscopic total gastrectomy a safe operation? A review of various anastomotic techniques and their outcomes. Surg Today 2015;45:549-58. [Crossref] [PubMed]
- Okabe H, Obama K, Tsunoda S, et al. Advantage of completely laparoscopic gastrectomy with linear stapled reconstruction: a long-term follow-up study. Ann Surg 2014;259:109-16. [Crossref] [PubMed]
- Okabe H, Obama K, Kan T, et al. Medial approach for laparoscopic total gastrectomy with splenic lymph node dissection. J Am Coll Surg 2010;211:e1-6. [Crossref] [PubMed]
- Hyung WJ, Lim JS, Song J, et al. Laparoscopic spleen-preserving splenic hilar lymph node dissection during total gastrectomy for gastric cancer. J Am Coll Surg 2008;207:e6-11. [Crossref] [PubMed]
- Guan G, Jiang W, Chen Z, et al. Early results of a modified splenic hilar lymphadenectomy in laparoscopy-assisted total gastrectomy for gastric cancer with stage cT1-2: a case-control study. Surg Endosc 2013;27:1923-31. [Crossref] [PubMed]
- Mou TY, Hu YF, Yu J, et al. Laparoscopic splenic hilum lymph node dissection for advanced proximal gastric cancer: a modified approach for pancreas- and spleen-preserving total gastrectomy. World J Gastroenterol 2013;19:4992-9. [Crossref] [PubMed]
- Huang CM, Zhang JR, Zheng CH, et al. A 346 case analysis for laparoscopic spleen-preserving no.10 lymph node dissection for proximal gastric cancer: a single center study. PLoS One 2014;9:e108480. [Crossref] [PubMed]
- Li P, Huang CM, Zheng CH, et al. Laparoscopic spleen-preserving splenic hilar lymphadenectomy in 108 consecutive patients with upper gastric cancer. World J Gastroenterol 2014;20:11376-83. [Crossref] [PubMed]
- Son T, Lee JH, Kim YM, et al. Robotic spleen-preserving total gastrectomy for gastric cancer: comparison with conventional laparoscopic procedure. Surg Endosc 2014;28:2606-15. [Crossref] [PubMed]
- Wang JB, Huang CM, Zheng CH, et al. Role of 3DCT in laparoscopic total gastrectomy with spleen-preserving splenic lymph node dissection. World J Gastroenterol 2014;20:4797-805. [Crossref] [PubMed]
- Lu J, Huang CM, Zheng CH, et al. Major perioperative complications in laparoscopic spleen-preserving total gastrectomy for gastric cancer: perspectives from a high-volume center. Surg Endosc 2016;30:1034-42. [Crossref] [PubMed]
- Kinoshita T, Shibasaki H, Enomoto N, et al. Laparoscopic splenic hilar lymph node dissection for proximal gastric cancer using integrated three-dimensional anatomic simulation software. Surg Endosc 2015. [Epub ahead of print]. [Crossref] [PubMed]
- Wang W, Liu Z, Xiong W, et al. Totally laparoscopic spleen-preserving splenic hilum lymph nodes dissection in radical total gastrectomy: an omnibearing method. Surg Endosc 2015. [Epub ahead of print]. [Crossref] [PubMed]
- Zheng CH, Xu M, Huang CM, et al. Anatomy and influence of the splenic artery in laparoscopic spleen-preserving splenic lymphadenectomy. World J Gastroenterol 2015;21:8389-97. [Crossref] [PubMed]
- Usui S, Tashiro M, Haruki S, et al. Spleen preservation versus splenectomy in laparoscopic total gastrectomy with D2 lymphadenectomy for gastric cancer: A comparison of short-term outcomes. Asian J Endosc Surg 2016;9:5-13. [Crossref] [PubMed]
- Nakata K, Nagai E, Ohuchida K, et al. Technical feasibility of laparoscopic total gastrectomy with splenectomy for gastric cancer: clinical short-term and long-term outcomes. Surg Endosc 2015;29:1817-22. [Crossref] [PubMed]
- Lee JH, Ahn SH. Laparoscopic total gastrectomy with D2 lymphadenectomy for advanced gastric cancer. World J Surg 2012;36:2394-9. [Crossref] [PubMed]
- Csendes A, Burdiles P, Rojas J, et al. A prospective randomized study comparing D2 total gastrectomy versus D2 total gastrectomy plus splenectomy in 187 patients with gastric carcinoma. Surgery 2002;131:401-7. [Crossref] [PubMed]
- Yu W, Choi GS, Chung HY. Randomized clinical trial of splenectomy versus splenic preservation in patients with proximal gastric cancer. Br J Surg 2006;93:559-63. [Crossref] [PubMed]
- Oh SJ, Hyung WJ, Li C, et al. The effect of spleen-preserving lymphadenectomy on surgical outcomes of locally advanced proximal gastric cancer. J Surg Oncol 2009;99:275-80. [Crossref] [PubMed]
- Yang K, Chen XZ, Hu JK, et al. Effectiveness and safety of splenectomy for gastric carcinoma: a meta-analysis. World J Gastroenterol 2009;15:5352-9. [Crossref] [PubMed]
- Sobin LH, Gospodarowicz MK, Wittekind C, editors. TNM Classification of Malignant Tumours. 7th Edition. New York: Wiley-Blackwell, 2009:73-7.
- Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205-13. [Crossref] [PubMed]
- Ajani JA, Bentrem DJ, Besh S, et al. Gastric cancer, version 2.2013: featured updates to the NCCN Guidelines. J Natl Compr Canc Netw 2013;11:531-46. [PubMed]
- Sano T, Yamamoto S, Sasako M, et al. Randomized controlled trial to evaluate splenectomy in total gastrectomy for proximal gastric carcinoma: Japan clinical oncology group study JCOG 0110-MF. Jpn J Clin Oncol 2002;32:363-4. [Crossref] [PubMed]
- Sano T, Sasako M, Mizusawa J, et al. Randomized controlled trial to evaluate splenectomy in total gastrectomy for proximal gastric carcinoma (JCOG0110): Final survival analysis. J Clin Oncol 2015;33: abstr 103.
- Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer 2011;14:113-23. [Crossref] [PubMed]
Cite this article as: Hosogi H, Okabe H, Shinohara H, Tsunoda S, Hisamori S, Sakai Y. Laparoscopic splenic hilar lymphadenectomy for advanced gastric cancer. Transl Gastroenterol Hepatol 2016;1:30.


