Minimally invasive function-preserving surgery based on sentinel node concept in early gastric cancer
Introduction
Many patients with early gastric cancer are currently treated with advanced laparoscopic gastrectomy procedures, such as laparoscopy-assisted distal gastrectomy (LADG) and laparoscopy-assisted total gastrectomy with standard lymph node dissection in Asian countries (1-4). Advanced laparoscopic gastrectomy contributes to both better esthetics and early postoperative recovery (5). However, patients’ quality of life (QOL) is mainly affected by late phase complications including dumping syndrome and body weight loss resulting from oral intake disturbance. Therefore, both minimal invasiveness for early phase recovery by laparoscopic surgery and additional late phase function-preserving gastric cancer surgery should be carefully considered in patients indicated for these procedures.
Function-preserving gastrectomy such as partial gastrectomy, segmental gastrectomy, and proximal gastrectomy with limited lymph node dissection is known to improve postoperative late phase function. However, a certain incidence of skip metastasis in the 2nd or 3rd compartment of regional lymph nodes remains an obstacle to the wider application of these procedures. To overcome these issues, the concept of sentinel node (SN) mapping may become a novel diagnostic tool for the identification of clinically undetectable lymph node metastasis in patients with early gastric cancer.
The clinical application of SN mapping for early gastric cancer has been controversial for years. However, single institutional results, including ours and those from a recent multicenter trial of SN mapping for early gastric cancer, are considered acceptable in terms of the SN detection rate and accuracy of determination of lymph node status (6,7). On the basis of these results, we are developing a novel, minimally invasive function-preserving gastrectomy technique combined with SN mapping.
Laparoscopic SN mapping procedures
A dual-tracer method that utilizes radioactive colloids and blue dyes is currently considered the most reliable method for the stable detection of SNs in patients with early gastric cancer (7,8). An accumulation of radioactive colloids facilitates the identification of SNs even in resected specimens by using a hand-held gamma probe, and the blue dye is effective for intraoperative visualization of lymphatic flow, even during laparoscopic surgery. Technetium-99m tin colloid, technetium-99m sulfur colloid, and technetium-99m antimony sulfur colloid are preferentially used as radioactive tracers. Isosulfan blue and indocyanine green (ICG) are the currently preferred choices as dye tracers.
In our institution, patients with clinical T1 (or T2) tumors, primary lesions less than 4 cm in diameter, and clinical N0 gastric cancer, undergo SN mapping and biopsy. In our procedures, 2.0 mL (150 MBq) of technetium-99m tin colloid solution is injected the day before surgery into four quadrants of the submucosal layer of the primary tumor site using an endoscopic puncture needle. Endoscopic injections facilitate accurate tracer injection. Technetium-99m tin colloid with relatively large particle size accumulates in the SNs after local administration.
The blue dye is injected into four quadrants of the submucosal layer of the primary site using an endoscopic puncture needle at the beginning of surgery. Blue lymphatic vessels and blue-stained nodes can be identified by laparoscopy within 15 min after the injection of the blue dye. Simultaneously, a hand-held gamma probe is used to locate the radioactive SN, similar to esophageal SN mapping. Intraoperative gamma probing is feasible in laparoscopic gastrectomy using a special gamma detector introducible from trocar ports.
For intraoperative SN sampling, the pick-up method is well established for the detection of melanoma and breast cancer. However, it is recommended that the clinical application of intraoperative SN sampling for gastric cancer should include sentinel lymphatic basin dissection, which is a sort of focused lymph node dissection involving hot and blue nodes (7,8). The gastric lymphatic basins were considered to be divided in the following five directions along the main arteries: left gastric artery area, right gastric artery area, left gastroepiploic artery area, right gastroepiploic artery area, and posterior gastric artery area (9).
ICG is known to have excitation and fluoresce wavelengths in the near-infrared range (10). Till date, some investigators have used infrared ray electronic endoscopy (IREE) to demonstrate the clinical utility of intraoperative ICG infrared imaging as a new tracer for laparoscopic SN biopsy (11,12). IREE might be a useful tool to improve visualization of ICG-stained lymphatic vessels and SNs even in the fat tissues. More recently, ICG fluorescence imaging has been developed as another promising novel technique for SN mapping (13,14). SN could be clearly visualized by ICG fluorescence imaging compared to the naked eye. Further studies would be needed to evaluate the clinical efficacy of ICG infrared or fluorescence imaging and to compare those with radio-guided methods in prospective studies. However these new technologies might revolutionize the SN mapping procedures not only in gastric cancer but also in many other solid tumors.
Results of SN mapping for gastric cancer
To date, more than 50 single institutional studies have demonstrated acceptable outcomes of SN mapping for early gastric cancer in terms of the SN detection rate (90–100%) and accuracy (85–100%) of determination of lymph node status; these outcomes are comparable to those of SN mapping for melanoma and breast cancer (8). Recently, Wang et al. reported a systematic review that evaluated the diagnostic value of SN biopsy for gastric cancer (14). The results of their large-scale meta-analysis, which included 38 relevant studies with 2,128 patients, demonstrated that the SN detection rate and accuracy of prediction of lymph node metastasis based on SN status were 94% and 92%, respectively (14). They concluded that the SN concept is technically feasible for gastric cancer, especially cases with early T stage (T1), with the use of combined tracers and submucosal injection methods during the SN biopsy procedures.
Our group in the Japan recently conducted a multicenter prospective trial of SN mapping using a dual-tracer method with a radioactive colloid and blue dye (7). In the trial, SN mapping was performed between 2004 and 2008 for approximately 400 patients with early gastric cancer at 12 comprehensive hospitals, including our institution. Eligibility criteria were that patients had cT1N0M0 or cT2N0M0 single tumor with diameter of primary lesion less than 4 cm, without any previous treatments. As results, the SN detection rate was 98% and the accuracy of determination of metastatic status was 99% (7). The results of that clinical trial are expected to provide us with perspectives on the future of SN navigation surgery for early gastric cancer.
Clinical application of laparoscopic SN mapping for early gastric cancer
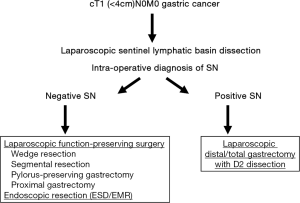
The distribution of sentinel lymphatic basins and the pathological status of SNs would be useful in deciding on the minimized extent of gastric resection and in avoiding the universal application of distal or total gastrectomy with D2 dissection. Appropriate indications for laparoscopic surgeries such as partial (wedge) resection, segmental gastrectomy, pylorus-preserving gastrectomy, and proximal gastrectomy (LAPG) for cT1N0 gastric cancer could be individually determined on the basis of SN status (Figures 1,2) (15,16). Earlier recovery after surgery and preservation of QOL in the late phase can be achieved by laparoscopic limited gastrectomy with SN navigation. Our study group in Japan currently started the multicenter prospective trial which will evaluate the function-preserving gastrectomy with SN mapping in terms of long-term survival and patients’ QOL as the next step.
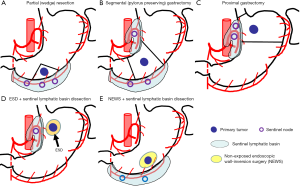
A combination of laparoscopic SN biopsy and endoscopic mucosal resection (EMR)/endoscopic submucosal dissection (ESD) for early gastric cancer is another attractive option as a novel, whole stomach-preserved, minimally invasive approach. If all SNs are pathologically negative for cancer metastasis, theoretically, EMR/ESD instead of gastrectomy may be sufficient for the curative resection of cT1 gastric cancer beyond the EMR criteria (Figure 2E) (17,18). However, further studies are required to verify the safety and effectiveness of combined treatments involving laparoscopic SN biopsy and EMR/ESD.
Nowadays, LADG or LAPG are frequently applied to the patients with early gastric cancer according to the results of pathological assessment of primary tumor resected by EMR/ESD in clinical practices. To date, it has not been clarified whether the SN mapping is feasible even after EMR/ESD. One of the most important issues is whether lymphatic flow from the primary tumor to the original SNs may change after EMR/ESD. In our preliminary study, however, at least the sentinel lymphatic basin is not markedly affected by previous EMR/ESD (17,18). Modified gastrectomy according to SN distribution and metastatic status might be feasible even for the patients who underwent EMR/ESD prior to surgery.
Non-exposed endoscopic wall-inversion surgery (NEWS) plus SN mapping
In current function-preserving surgeries such as laparoscopic local resection or segmental gastrectomy, the approach of gastrectomy is only from the outside of the stomach, in which the demarcation line of the tumor cannot be visualized at the phase of resection. Therefore, the surgeons cannot avoid a wider resection of the stomach than is desired to prevent a positive surgical margin. The recent appearance of a new technique, referred to as NEWS is a technique of full thickness partial resection, which can minimize the extent of gastric resection using endoscopic and laparoscopic surgery without transluminal access mainly designed to treat gastric cancer. We have been accumulating cases of NEWS with SN biopsy for early gastric cancer with the risk of lymph node metastasis in the clinical trial (19,20).
In briefly, after placing mucosal markings, ICG was injected endoscopically into the submucosa around the lesion to examine SNs (Figure 3) (19). The SN basin including hot or stained SNs was dissected, and an intraoperative pathological diagnosis confirmed that no metastasis had occurred. Subsequently, NEWS was performed for the primary lesion. Serosal markings were placed laparoscopically, submucosal injection was added endoscopically, and circumferential sero-muscular incision and suturing were performed laparoscopically, with the lesion inverted toward the inside of the stomach. Finally, the circumferential mucosal incision was performed, and the lesion was retrieved perorally.
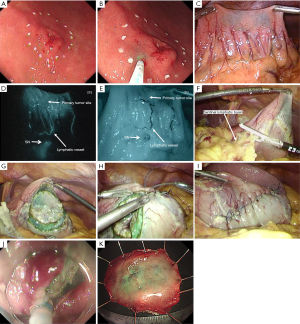
The NEWS combined with the SN biopsy can minimize not only the area of lymphadenectomy, but also the extent of gastric resection as partial gastrectomy for patients with SN-negative for metastasis (19). Furthermore, NEWS does not need intentional perforation, which enables us to apply this technique to cancers without a risk of iatrogenic dissemination. The combination of NEWS with SN biopsy is expected to become a promising, ideal minimally invasive, function-preserving surgery to cure cases of cN0 early gastric cancer.
For early stage gastric cancer, for which a better prognosis can be achieved through conventional surgical approaches, the establishment of individualized, minimally invasive treatments that may retain the patients’ QOL should be the next surgical challenge. Although further studies are needed for careful validation, function preserving gastrectomy based on SN navigation could be a promising strategy to achieve this goal.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Kitano S, Iso Y, Moriyama M, et al. Laparoscopy-assisted Billroth I gastrectomy. Surg Laparosc Endosc 1994;4:146-8. [PubMed]
- Adachi Y, Shiraishi N, Shiromizu A, et al. Laparoscopy-assisted Billroth I gastrectomy compared with conventional open gastrectomy. Arch Surg 2000;135:806-10. [Crossref] [PubMed]
- Shinohara T, Kanaya S, Taniguchi K, et al. Laparoscopic total gastrectomy with D2 lymph node dissection for gastric cancer. Arch Surg 2009;144:1138-42. [Crossref] [PubMed]
- Hur H, Jeon HM, Kim W. Laparoscopy-assisted distal gastrectomy with D2 lymphadenectomy for T2b advanced gastric cancers: three years' experience. J Surg Oncol 2008;98:515-9. [Crossref] [PubMed]
- Kim YW, Baik YH, Yun YH, et al. Improved quality of life outcomes after laparoscopy-assisted distal gastrectomy for early gastric cancer: results of a prospective randomized clinical trial. Ann Surg 2008;248:721-7. [Crossref] [PubMed]
- Kitagawa Y, Fujii H, Mukai M, et al. The role of the sentinel lymph node in gastrointestinal cancer. Surg Clin North Am 2000;80:1799-809. [Crossref] [PubMed]
- Kitagawa Y, Takeuchi H, Takagi Y, et al. Sentinel node mapping for gastric cancer: a prospective multicenter trial in Japan. J Clin Oncol 2013;31:3704-10. [Crossref] [PubMed]
- Takeuchi H, Kitagawa Y. New sentinel node mapping technologies for early gastric cancer. Ann Surg Oncol 2013;20:522-32. [Crossref] [PubMed]
- Kinami S, Fujimura T, Ojima E, et al. PTD classification: proposal for a new classification of gastric cancer location based on physiological lymphatic flow. Int J Clin Oncol 2008;13:320-9. [Crossref] [PubMed]
- Tajima Y, Murakami M, Yamazaki K, et al. Sentinel node mapping guided by indocyanine green fluorescence imaging during laparoscopic surgery in gastric cancer. Ann Surg Oncol 2010;17:1787-93. [Crossref] [PubMed]
- Ishikawa K, Yasuda K, Shiromizu A, et al. Laparoscopic sentinel node navigation achieved by infrared ray electronic endoscopy system in patients with gastric cancer. Surg Endosc 2007;21:1131-4. [Crossref] [PubMed]
- Nimura H, Narimiya N, Mitsumori N, et al. Infrared ray electronic endoscopy combined with indocyanine green injection for detection of sentinel nodes of patients with gastric cancer. Br J Surg 2004;91:575-9. [Crossref] [PubMed]
- Miyashiro I, Miyoshi N, Hiratsuka M, et al. Detection of sentinel node in gastric cancer surgery by indocyanine green fluorescence imaging: comparison with infrared imaging. Ann Surg Oncol 2008;15:1640-3. [Crossref] [PubMed]
- Wang Z, Dong ZY, Chen JQ, et al. Diagnostic value of sentinel lymph node biopsy in gastric cancer: a meta-analysis. Ann Surg Oncol 2012;19:1541-50. [Crossref] [PubMed]
- Takeuchi H, Saikawa Y, Kitagawa Y. Laparoscopic sentinel node navigation surgery for early gastric cancer. Asian J Endosc Surg 2009;2:13-7. [Crossref]
- Takeuchi H, Oyama T, Kamiya S, et al. Laparoscopy-assisted proximal gastrectomy with sentinel node mapping for early gastric cancer. World J Surg 2011;35:2463-71. [Crossref] [PubMed]
- Takeuchi H, Kitagawa Y. Sentinel node navigation surgery in patients with early gastric cancer. Dig Surg 2013;30:104-11. [Crossref] [PubMed]
- Mayanagi S, Takeuchi H, Kamiya S, et al. Suitability of sentinel node mapping as an index of metastasis in early gastric cancer following endoscopic resection. Ann Surg Oncol 2014;21:2987-93. [Crossref] [PubMed]
- Goto O, Takeuchi H, Kawakubo H, et al. First case of non-exposed endoscopic wall-inversion surgery with sentinel node basin dissection for early gastric cancer. Gastric Cancer 2015;18:434-9. [Crossref] [PubMed]
- Takeuchi H, Kitagawa Y. Sentinel lymph node biopsy in gastric cancer. Cancer J 2015;21:21-4. [Crossref] [PubMed]
Cite this article as: Takeuchi H, Kitagawa Y. Minimally invasive function-preserving surgery based on sentinel node concept in early gastric cancer. Transl Gastroenterol Hepatol 2016;1:23.


