Review of management options for pancreatic pseudocysts
Introduction
Throughout pancreatic surgery history, the term pancreatic pseudocyst (PP) has been a field of debate between surgeons, gastroenterologists and radiologists, regarding their origin, evolution and management. Nowadays, PPs are considered as fluid collections in the peripancreatic tissue, which occasionally can be found partly or totally inside the pancreatic parenchyma. Imaging of PP shows fluid surrounded by a well-defined wall containing no solid material (1). However, the rather rare appearance of pseudocysts, along with the lack of solid guidelines regarding their treatment, continues to raise new dilemmas and questions about their optimal management.
Since the first surgical internal drainage in 1921 (2), surgery remained until recently the cornerstone in the management of PP. In the recent years, minimally invasive techniques, including laparoscopic procedures, endoscopic and radiology-guided interventions, have increased the available options in the treatment of PP. However, inconsistence of definitions but mainly the lack of large series of patients, have limited the amount of randomized control trials about diagnosis and treatment of PP.
Consequently, absence of level A evidence cannot provide safe conclusions on the optimal method of treatment, in this sometime difficult to understand and treat pancreatic entity.
The aim of this review is to provide the latest evidence found in the literature, regarding the management of PPs. A short review on the classification, etiology and diagnosis, which could aid the treatment algorithm, will be presented. Moreover, available surgical techniques and results will be described, along with the novel techniques in terms of endoscopic and percutaneous management. Finally, all available treatment strategies will be compared in order to find the optimal treatment or combination of treatment methods.
Classification
In support of the complexity and confusion in the management of PP, there are numerous classifications based on time of onset, morphological and clinical characteristics. Sarles et al. provided one of the first classifications of pseudocysts based on the underlying existence of acute or chronic pancreatitis (3). In 1991, D’Edigio et al., included in their classification the underlying chronic or acute pancreatitis, the pancreatic ductal anatomy and the presence of communication between the cyst and the ducts, defining three distinct types of pseudocysts (4). Other classifications are based upon the extension of necrosis or entirely upon the pancreatic duct anatomy (5). Finally the Atlanta classification in 1992 and especially its revision in 2012, tried to distinguish pseudocysts, from acute peripancreatic fluid collections and acute necrotic collections, stating that the development of pseudocyst in the setting of acute pancreatitis is rare (1).
Etiology and incidence
The term PP refers specifically to a fluid collection in the peripancreatic tissue, which usually is rich of pancreatic juice. Disruption of the main pancreatic duct or its side branches seems to be the main etiological factor for the creation of a pseudocyst, especially in the setting of acute pancreatitis. Subsequent leakage of pancreatic juice leads to a localized accumulation of clear fluid, forming a collection, usually after 4 weeks (1).
On the other hand, pathogenesis of pseudocysts formation following chronic pancreatitis is not well understood. It seems that apart from the acute fluid exacerbation, blockage of the main pancreatic duct from a protein plug or calculus can lead to the pseudocyst formation (6). Connection between a pseudocyst and the main pancreatic duct can be demonstrated in two thirds of the patients, whereas in the rest, the connection is sealed probably from an inflammatory reaction (6).
Pseudocysts arise in the setting of acute or chronic pancreatitis, but also after pancreatic trauma either from external injury or during surgery such as gastric resection. They can be asymptomatic or may present with pain, vomiting, nausea or even upper gastrointestinal bleeding (7). The incidence of pseudocysts is extremely low ranging from 1.6–4.5% per 100,000 adults per year (8). The prevalence of PPs is ranging from 10% to 26% in acute pancreatitis and 20–40% in chronic pancreatitis (9). Patients with chronic pancreatitis present with a pseudocyst more often after alcohol induced chronic pancreatitis (70–78%), followed by idiopathic chronic pancreatitis (6–16%) and biliary chronic pancreatitis (6–8%) (8,10).
Management of PPs
Management of PPs comprises two major aspects: conservative management with supportive care and active definitive care with any type of intervention. Apart from the well-established watchful follow up management and the traditional surgical drainage (SD), more recently, percutaneous and endoscopic drainage (ED) techniques have been evaluated with promising results. Nowadays, modern methods of diagnosis and detailed evaluation of the anatomy of pseudocysts, along side with the knowledge that they can possibly resolve on their own, can safely lead a physician to the optimal choice of treatment.
Diagnosis and prognosis
Diagnosis can only be set with medical imaging. Transabdominal ultrasound with its portability and ease of access is one of the most frequently used diagnostic tools in evaluating a pseudocyst. However, it is operator-dependent, with not reproducible results and imaging limitations such as overlying bowel-gas. Its sensitivity in the detection of PPs ranges from 70–90% (6,11). Computer tomography (CT), visualizing a thick-walled and clear fluid-filled mass adjacent to the pancreas, in a patient with history of acute or chronic pancreatitis, is almost pathognomonic for PP. Moreover, CT is also useful in the differential diagnosis between pseudocysts and walled-off necrosis, offering recognition of solid components and debris (11). Sensitivity of CT in diagnosing pseudocysts ranges from 90–100% (8). Magnetic resonance imaging (MRI) and magnetic resonance cholangiopancreatography (MRCP), are the most accurate and sensitive diagnostic tools, in order to evaluate the anatomy of the pancreatic duct (8). Moreover, exclusion of pancreatic duct disruption and therefore establishment of possible communication with a PP can be more safely done with MRI-MRCP (12). Lastly, MRI provides more information regarding the prediction of possible drainage with sensitivity and specificity of 100% compared with CT, 25% and 100% and ultrasound, 88% and 54%, respectively (11). Endoscopic retrograde cholangiopancreatography (ERCP) remains the gold standard in the diagnosis of pancreatic duct disruption, but is limited by its invasive nature and possible complications and can be more useful for therapeutic purpose. Finally, endoscopic ultrasound (EUS) has more sensitivity than CT in imaging possible solid debris, and thus excluding the presence of pseudocyst (11). In general, EUS has the highest sensitivity (93–100%) and specificity (92–98%) in the distinction of PP from acute fluid collections.
Spontaneous resolution of pseudocyst is not uncommon, especially for those presented after an episode of acute pancreatitis, ranging from 8–70% (13). There are several factors decreasing the possibility of spontaneous resolution, like the presence of multiple cysts, location in the tail of the pancreas, communication with the main pancreatic duct and co-existence of stricture and increasing in size during follow up (13,14). On the other hand, PPs due to chronic pancreatitis are rarely resolving spontaneously, mainly due to abnormalities of the pancreatic duct and strictures produced by calcification and fibrosis (13,14).
Supportive care
Based on the possibility that spontaneous resolution can occur, the gold standard for the treatment of uncomplicated PPs, is conservative treatment. Moreover, even without resolution, most pseudocysts if not enlarging, rarely cause any significant symptom. In patients that can tolerate oral intake, enteral low fat diet is recommended, along with the addition of analgesics and antiemetic’s, if needed (6). Patients are frequently monitored by different imaging modalities, for size comparison and presence of complications, although the time intervals are not universally standardized.
Interventional techniques
Uncomplicated and asymptomatic pseudocysts, remaining stable or even diminishing in size, can be safely managed with a non-operative approach. On the other hand, presence of symptoms like pain, discomfort, vomit or frequent admissions for intravenous fluid resuscitation, along with the development of complications like infection, bleeding or rupture in adjacent organs are indications for the implementation of interventional techniques (Table 1). The main types of interventions are the percutaneous drainage (PD), the ED and the SD or excision. Timing of intervention should be delayed up to 6 weeks from the pancreatitis episode, in the absence of life threatening events, in order for the pseudocyst wall to mature and become thicker, aiding in the success of any type of drainage (2).

Full table
PD
PD is achieved through the insertion of a drainage catheter into the PP, by ultrasound or CT guidance. Contrast injection in the cavity can demonstrate the size of the remaining PP, in order to aid the follow up (8). Indications for PD are the same as those for choosing an interventional technique e.g., complications and symptoms. However, PD is extremely helpful in cases of fragile patients, with severe comorbidities, that cannot tolerate any other surgical or endoscopic procedure (14). On the other hand, PD has severe limitations such as the scarcity of a safe access route, presence of active hemorrhage into the pseudocyst and demonstration of pancreatic duct obstruction or disruption, which will probably lead to the formation of pancreatic fistula (6,8,14). Furthermore, in cases without communication with the pancreatic duct, fine needle aspiration and evacuation, can be useful for diagnostic and therapeutic reasons, with minimum risk of complications (14).
There are several studies evaluating the effectiveness and results of PD when compared with ED and surgical intervention. In one of the largest studies, Morton and colleagues compared surgical and PD and concluded that SD has fewer complications, less inpatient mortality and reduced hospital stay (15). Similar results were found in the study of Heider and colleagues, where SD was superior to PD in terms of complications, mortality and duration of hospitalization (16). When compared with ED, percutaneous intervention seems to have similar percentages of success rates and clinical success, but PD has worse results in terms of hospital stay and rates of reintervention (9.8% vs. 42%, P=0.001) (17). In a recent study, ED was compared with PD in the treatment of pancreatic fluid collections and although ED was associated with higher procedural adverse effects, PD was inferior in terms of residual collections (20% vs. 53%), need for reintervention and need for SD (4% vs. 6%) (18). More recently, novel hybrid and endoscopic techniques which combine pseudocyst irrigation and along with pancreatic necrosectomy have been described and performed in specialized centers (19).
In conclusion, PD can be considered a safe alternative for therapeutic intervention, in patients with immature infected pseudocyst, in critically ill patients or those that cannot tolerate surgical or endoscopic procedures. However, its contraindications and high rates of reintervention and prolonged hospital stay are limitations that should be taken under evaluation when designing a treatment algorithm for patients with pseudocysts.
ED
Endoscopic techniques are based upon the knowledge, that a pseudocyst is not an independent structure, but a single space delineated between normal anatomic structures like the stomach, the duodenum and the transverse mesocolon. Stomach or duodenum wall usually serve as the wall of the pseudocyst itself, allowing enterostomies to be performed, without concern of potential empty spaces between the pseudocyst and the adjacent organs (14). ED can be performed either transpapillary drainage (TPD) or transmural (TSM), with or without the use of EUS.
TPD
TPD can be performed whenever there is a communication between the pseudocyst and the pancreatic duct, which can be demonstrated with ERCP and is also indicated in cysts that are too distant (>1 cm) from the gastrointestinal lumen to allow safe TSM drainage (20). With this technique a stent can be passed through the pancreatic duct into the pseudocyst. Moreover, if there is a ductal leak or structure, stenting can bridge the leak or dilate the stricture (21). TPD is usually successful in all patients with a resolution of pseudocysts ranging from 81–94%, while 6–39% will require surgery after 4 years of follow-up (14). Main reasons for failure of TPD are presence of chronic pancreatitis, distally located pseudocysts and multiple pancreatic duct strictures (14). However, one has to bear in mind that the majority of studies using TPD, definition of pseudocyst was not standardized, leading to severe bias in interpreting the main results.
Nowadays there is a trend in combining TPD with TSM drainage, although the literature is probably divergent regarding the results. Trevino et al., for example, showed that the combination of these techniques had a positive impact on the resolution of pseudocysts (22), while Yang et al. could not find any benefit from the combination of TPD and TSM over TSM alone (23). At the moment the use of TPD in the management of PP, alone or in combination with TSM, remains ambiguous.
TSM drainage
Conventional TSM drainage was first introduced in 1975 by Rogers et al. (24). It requires close proximity of the pseudocyst with the gastrointestinal lumen and endoscopic localization in the form of a visible luminal bulge (20). However, there some concerns about this method, especially for pseudocysts located in the tail of the pancreas and thus not producing a visible bulge and for cases with the interference of vessels and collaterals that increase the possibility of bleeding (25). Entry in the pseudocyst with the conventional TSM drainage is achieved either with the method of diathermic puncture or with the Seldinger technique (20). The fact that 42–48% of the pseudocysts do not produce a bulging sign (9), along with the need to access cysts that are in close proximity, but not attached to the gastrointestinal lumen, leaded investigators to add the EUS in the TSM drainage technique, as it was first described in 1992 by Grimm et al. (26). EUS-TSM drainage, allows the drainage of non-bulging pseudocysts, with a cyst-lumen distance of 1–1.5 cm, under direct visualization, avoiding complications like hemorrhage from gastric varices or any damage to normal pancreatic parenchyma (20).
Endoscopic TSM techniques produce excellent results, with a complete resolution of pseudocysts ranging from 71–95% of the cases and complications ranging from 0–37% (20). The main complications are lack of cyst resolution reaching 15% and bleeding with percentages ranging from 0–9%. Moreover, less frequent are immediate or delayed infection (0–8%) and retroperitoneal perforation (0–5%) (27,28). Bleeding can occur after puncture of gastric varices or pseudoaneurysms, while retroperitoneal perforation, a serious complication, which occurs in immature pseudocysts or lumen-cyst distances greater than 1.5 cm (20).
There are several studies comparing traditional TSM drainage with the newest EUS-TSM drainage. In the first randomized trial that compared the two techniques, Varadarajulu and colleagues found that the EUS-TSM resulted to 100% drainage success compared to only 33% with the classic TSM (P<0.001) (29). Furthermore, in the same study, all patients with failed drainage were successfully treated under EUS-guidance and 2 patients who underwent traditional ED suffered from severe bleeding (29). Park et al., in their randomized trial found that EUS-TSM drainage and traditional TSM drainage significantly differ only in terms of technical success (P=0.039), whereas no difference could be found in terms of complication rate (P=0.67), pseudocyst resolution (P=0.565) and long term clinical outcomes (P=0.696) (30). However, it should be noticed that in all cases that traditional drainage had failed, addition of EUS leaded to successful treatment, suggesting that EUS-TSM is the preferred method of choice, especially in non-bulging pseudocysts (30). Finally in a recent Cochrane review, no significant difference could be identified between traditional and EUS TSM drainage in terms of short term mortality or percentage of serious adverse events (31). However, EUS-TSM resulted to fewer additional interventions and shorter hospital stay (31).
In conclusion, ED results to nearly 100% successful drainage and resolution of pseudocysts. TPD is indicated especially when the pancreatic duct is disrupted and communicating with the pseudocyst and can be combined with TSM drainage. Conventional and EUS-TSM drainage seem to be more effective in the long term control of pseudocysts and are associated with a decreased percentage of serious complications. The two latter techniques do not significantly differ in terms of morbidity and mortality, but probably EUS-TSM is the preferred method especially in non-bulging pseudocysts.
SD
Historically, SD was the method of choice for PP drainage, with recurrence rates up to 5% and complication rates up to 30% (9). Cystogastrostomy, cystoduodenostomy and cystojejunostomy are the usual types of operations, performed either open or laparoscopically, creating an anastomosis between the GI tract lumen and the cyst, using suturing or stapling devices (32). However, with the evolving of endoscopic and percutaneous techniques, traditional SD has been limited in its indications. Nowadays, surgical resection is warranted for cases in which the other modalities of treatment have failed or cannot be performed, for cases of recurrent pseudocysts, when the diagnosis of cystic neoplasm cannot definitely be ruled out and finally for cases combined with bile duct or duodenal stenosis (8,14).
Development of laparoscopic techniques, along with evolving technology, has safely permitted the broad use of laparoscopy in the internal drainage of pseudocysts, following the same principles with open surgery, with the most frequent type of procedure being the cystogastrostomy. Pseudocysts can be drained internally via the endogastric, extragastric or transgastric approaches (33,34). In a systematic review of the literature 10 years ago, laparoscopic drainage was associated with 98.3% achievement of drainage, 2.5% recurrence, 5.7 days mean hospital day and <2% complication rates (35). Finally the first comparative study between open and laparoscopic drainage, revealed that the latter was associated with reduced operative time, fewer operative complications and decreased hospital stay (36).
In one of the first studies comparing open, laparoscopic and ED, Melman et al. found that there were no significant differences between the three approaches in terms of Grade 2 or greater complications (37). Furthermore, primary success was significantly better with open and laparoscopic approach than the endoscopic, but repeated endoscopic procedures leaded to similar, not significant, results among the three approaches (37). These results were repeated by another study in 2008, where a comparison between EUS and open drainage showed no differences in morbidity or treatment success (38). In one randomized trial, comparing endoscopic and surgical cystogastrostomy, no significant difference was identified in terms of treatment success, complications or re-interventions (39). However, ED was associated with shorter hospital stay (2 vs. 6 days) and fewer total mean cost ($7011 vs. 15.052) (39). The main comparative studies between percutaneous, endoscopic and SD are presented in Table 2.
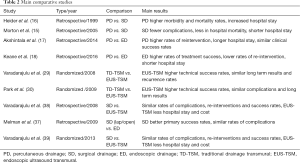
Full table
Finally, although rarely, in cases of multiple pseudocysts and those with associated pseudoaneurysms, biliary or severe duodenal obstruction and underlying symptomatic chronic pancreatitis, resection along with pancreatectomy could be an option (14).
In conclusion, SD of pseudocysts, open or laparoscopic, is a safe alternative to less invasive methods of drainage. The major advantage of SD is the creation of a wider stoma for drainage and possible debridement in cases of underlying pancreatic or peripancreatic necrosis.
Conclusions
Management of PPs has evolved over the years, from an aggressive approach, to a more conservative management. In cases of symptomatic or complicated pseudocysts, a plethora of techniques and types of drainage can lead to almost 100% primary and overall success of pseudocyst drainage. PD can be offered in fragile patients or those that cannot tolerate other modalities of treatment with good results. Endoscopic procedures, especially after the introduction of EUS, seem to be safe, effective and probably will become, if not yet, the preferred method of choice. Finally, the historical gold standard of SD, has proven its efficacy and with the addition of laparoscopy, remains a reliable method especially in large and complicated pseudocysts.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Banks PA, Bollen TL, Dervenis C, et al. Classification of acute pancreatitis--2012: revision of the Atlanta classification and definitions by international consensus. Gut 2013;62:102-11. [Crossref] [PubMed]
- Parks RW, Tzovaras G, Diamond T, et al. Management of pancreatic pseudocysts. Ann R Coll Surg Engl 2000;82:383-7. [PubMed]
- Sarles H, Martin M, Camatte R, et al. The separation of the pancreatites: the pseudocysts of acute pancreatitis and of chronic pancreatitis. Presse Med 1963;71:237-40. [PubMed]
- D'Egidio A, Schein M. Pancreatic pseudocysts: a proposed classification and its management implications. Br J Surg 1991;78:981-4. [Crossref] [PubMed]
- Nealon WH, Walser E. Main pancreatic ductal anatomy can direct choice of modality for treating pancreatic pseudocysts (surgery versus percutaneous drainage). Ann Surg 2002;235:751-8. [Crossref] [PubMed]
- Habashi S, Draganov PV. Pancreatic pseudocyst. World J Gastroenterol 2009;15:38-47. [Crossref] [PubMed]
- Pan G, Wan MH, Xie KL, et al. Classification and Management of Pancreatic Pseudocysts. Medicine (Baltimore) 2015;94:e960. [Crossref] [PubMed]
- Khanna AK, Tiwary SK, Kumar P. Pancreatic pseudocyst: therapeutic dilemma. Int J Inflam 2012;2012:279476.
- Andalib I, Dawod E, Kahaleh M. Modern Management of Pancreatic Fluid Collections. J Clin Gastroenterol 2018;52:97-104. [PubMed]
- Aghdassi AA, Mayerle J, Kraft M, et al. Pancreatic pseudocysts--when and how to treat? HPB (Oxford) 2006;8:432-41. [Crossref] [PubMed]
- Dhaka N, Samanta J, Kochhar S, et al. Pancreatic fluid collections: What is the ideal imaging technique? World J Gastroenterol 2015;21:13403-10. [Crossref] [PubMed]
- Kamal A, Singh VK, Akshintala VS, et al. CT and MRI assessment of symptomatic organized pancreatic fluid collections and pancreatic duct disruption: an interreader variability study using the revised Atlanta classification 2012. Abdom Imaging 2015;40:1608-16. [Crossref] [PubMed]
- Andren-Sandberg A, Dervenis C. Pancreatic pseudocysts in the 21st century. Part II: natural history. JOP 2004;5:64-70. [PubMed]
- Andren-Sandberg A, Ansorge C, Eiriksson K, et al. Treatment of pancreatic pseudocysts. Scand J Surg 2005;94:165-75. [Crossref] [PubMed]
- Morton JM, Brown A, Galanko JA, et al. A national comparison of surgical versus percutaneous drainage of pancreatic pseudocysts: 1997-2001. J Gastrointest Surg 2005;9:15-20; discussion-1.
- Heider R, Meyer AA, Galanko JA, et al. Percutaneous drainage of pancreatic pseudocysts is associated with a higher failure rate than surgical treatment in unselected patients. Ann Surg 1999;229:781-7. [Crossref] [PubMed]
- Akshintala VS, Saxena P, Zaheer A, et al. A comparative evaluation of outcomes of endoscopic versus percutaneous drainage for symptomatic pancreatic pseudocysts. Gastrointest Endosc 2014;79:921-8. [Crossref] [PubMed]
- Keane MG, Sze SF, Cieplik N, et al. Endoscopic versus percutaneous drainage of symptomatic pancreatic fluid collections: a 14-year experience from a tertiary hepatobiliary centre. Surg Endosc 2016;30:3730-40. [Crossref] [PubMed]
- Baron TH, Kozarek RA. Endotherapy for organized pancreatic necrosis: perspectives after 20 years. Clin Gastroenterol Hepatol 2012;10:1202-7. [Crossref] [PubMed]
- Ge PS, Weizmann M, Watson RR. Pancreatic Pseudocysts: Advances in Endoscopic Management. Gastroenterol Clin North Am 2016;45:9-27. [Crossref] [PubMed]
- Varadarajulu S, Noone TC, Tutuian R, et al. Predictors of outcome in pancreatic duct disruption managed by endoscopic transpapillary stent placement. Gastrointest Endosc 2005;61:568-75. [Crossref] [PubMed]
- Trevino JM, Tamhane A, Varadarajulu S. Successful stenting in ductal disruption favorably impacts treatment outcomes in patients undergoing transmural drainage of peripancreatic fluid collections. J Gastroenterol Hepatol 2010;25:526-31. [Crossref] [PubMed]
- Yang D, Amin S, Gonzalez S, et al. Transpapillary drainage has no added benefit on treatment outcomes in patients undergoing EUS-guided transmural drainage of pancreatic pseudocysts: a large multicenter study. Gastrointest Endosc 2016;83:720-9. [Crossref] [PubMed]
- Rogers BH, Cicurel NJ, Seed RW. Transgastric needle aspiration of pancreatic pseudocyst through an endoscope. Gastrointest Endosc 1975;21:133-4. [Crossref] [PubMed]
- Nabi Z, Basha J, Reddy DN. Endoscopic management of pancreatic fluid collections-revisited. World J Gastroenterol 2017;23:2660-72. [Crossref] [PubMed]
- Grimm H, Binmoeller KF, Soehendra N. Endosonography-guided drainage of a pancreatic pseudocyst. Gastrointest Endosc 1992;38:170-1. [Crossref] [PubMed]
- Cahen D, Rauws E, Fockens P, et al. Endoscopic drainage of pancreatic pseudocysts: long-term outcome and procedural factors associated with safe and successful treatment. Endoscopy 2005;37:977-83. [Crossref] [PubMed]
- Kahaleh M, Shami VM, Conaway MR, et al. Endoscopic ultrasound drainage of pancreatic pseudocyst: a prospective comparison with conventional endoscopic drainage. Endoscopy 2006;38:355-9. [Crossref] [PubMed]
- Varadarajulu S, Christein JD, Tamhane A, et al. Prospective randomized trial comparing EUS and EGD for transmural drainage of pancreatic pseudocysts (with videos). Gastrointest Endosc 2008;68:1102-11. [Crossref] [PubMed]
- Park DH, Lee SS, Moon SH, et al. Endoscopic ultrasound-guided versus conventional transmural drainage for pancreatic pseudocysts: a prospective randomized trial. Endoscopy 2009;41:842-8. [Crossref] [PubMed]
- Gurusamy KS, Pallari E, Hawkins N, et al. Management strategies for pancreatic pseudocysts. Cochrane Database Syst Rev 2016;4:CD011392. [PubMed]
- Tyberg A, Karia K, Gabr M, et al. Management of pancreatic fluid collections: A comprehensive review of the literature. World J Gastroenterol 2016;22:2256-70. [Crossref] [PubMed]
- Bhattacharya D, Ammori BJ. Minimally invasive approaches to the management of pancreatic pseudocysts: review of the literature. Surg Laparosc Endosc Percutan Tech 2003;13:141-8. [Crossref] [PubMed]
- Zerem E, Hauser G, Loga-Zec S, et al. Minimally invasive treatment of pancreatic pseudocysts. World J Gastroenterol 2015;21:6850-60. [Crossref] [PubMed]
- Aljarabah M, Ammori BJ. Laparoscopic and endoscopic approaches for drainage of pancreatic pseudocysts: a systematic review of published series. Surg Endosc 2007;21:1936-44. [Crossref] [PubMed]
- Khaled YS, Malde DJ, Packer J, et al. Laparoscopic versus open cystgastrostomy for pancreatic pseudocysts: a case-matched comparative study. J Hepatobiliary Pancreat Sci 2014;21:818-23. [Crossref] [PubMed]
- Melman L, Azar R, Beddow K, et al. Primary and overall success rates for clinical outcomes after laparoscopic, endoscopic, and open pancreatic cystgastrostomy for pancreatic pseudocysts. Surg Endosc 2009;23:267-71. [Crossref] [PubMed]
- Varadarajulu S, Lopes TL, Wilcox CM, et al. EUS versus surgical cyst-gastrostomy for management of pancreatic pseudocysts. Gastrointest Endosc 2008;68:649-55. [Crossref] [PubMed]
- Varadarajulu S, Bang JY, Sutton BS, et al. Equal efficacy of endoscopic and surgical cystogastrostomy for pancreatic pseudocyst drainage in a randomized trial. Gastroenterology 2013;145:583-90.e1. [Crossref] [PubMed]
Cite this article as: Agalianos C, Passas I, Sideris I, Davides D, Dervenis C. Review of management options for pancreatic pseudocysts. Transl Gastroenterol Hepatol 2018;3:18.


